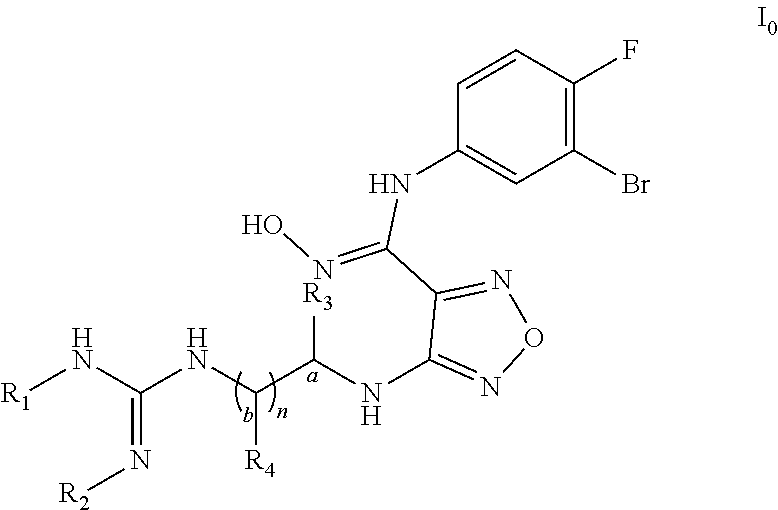
Imino-urea derivatives
a technology of urea and derivatives, applied in the field of medicine, can solve the problems of unsatisfactory clinical effects, mental illnesses such as depression and anxiety, and the level of 5-hydroxytryptamine is still low
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0088]
[0089]Compound 1 (903 mg, 10 mmol) was dissolved in acetone (10 mL), then potassium carbonate (2.76 g, 20 mmol) was added at room temperature, stirred at room temperature for 0.5 h, added dropwise with benzenesulfonyl chloride, stirred at room temperature overnight, quenched with water, and the resultant was extracted with ethyl acetate, dried over anhydrous sodium sulfate, and concentrated to obtain 900 mg of a white powder.
[0090]Compound 2 (230 mg, 1 mmol) was dissolved in acetonitrile (5 mL), stirred at room temperature for 0.5 h, then added with compound 2a (320 mg, 2 mmol), heated at 60° C. for 24 h, and the resultant was directly evaporated to dryness under reduced pressure, and subjected to purification by pre-HPLC to obtain 210 mg of the desired product.
[0091]Compound 3 (171 mg, 0.5 mmol) was dissolved in dichloromethane (10 mL), stirred at room temperature for 5 min, added with trifluoroacetic acid (5 mL), reacted at room temperature for 2 h, and then the resultant wa...
example 10
[0097]
[0098]Compound 1a (682 mg, 2 mmol) was dissolved in trifluoroacetic acid (13 mL), then 30% H2O2 was added dropwise at room temperature, the mixture was reacted overnight at 50° C., and the reaction solution changed from turbid to clear yellow. After the reaction was completed, the reaction was quenched with a saturated sodium sulfite solution. After KI starch test paper showed clolorless, the resultant was extracted with ethyl acetate (50 mL*2), and the organic phase was dried over anhydrous sodium sulfate, concentrated, and then subjected to purification by column chromatography (petroleum ether:ethyl acetate=1:1) to obtain a pale yellow solid 2a (500 mg, yield 67%).
[0099]Ethylenediamine 1 (30 mg, 0.5 mmol) was added to a solution of Compound 2a (170 mg, 0.5 mmol) in tetrahydrofuran (10 mL), then 1N NaOH (0.4 mL) was added, the reaction solution was stirred at room temperature for 0.5 h, and the reaction solution was directly used to prepare and obtain Compound 2 (120 mg).
[01...
example 11
[0102]
[0103]1,3-Diaminopropane (35 mg, 0.5 mmol) was added to a solution of Compound 1 (170 mg, 0.5 mmol, the synthesis method of which was referred to the synthesis of Compound 2a in XSD3-047 Final report) in tetrahydrofuran (10 mL), the reaction solution was stirred at room temperature for 0.5 h, and the reaction solution was directly used to prepare and obtain the desired product of 130 mg.
[0104]A mixture solution of Compound 2 (130 mg, 0.33 mmol) and Compound 1a (100 mg, 0.33 mmol) in methanol (10 mL) was stirred at room temperature overnight, the reaction solution was concentrated and subjected to purification by column chromatography (petroleum ether:ethyl acetate=1:1) to obtain Compound 3 (78 mg, 45%).
[0105]Trifluoroacetic acid (0.6 mL) was added to a solution of Compound 3 (31 mg, 0.05 mmol) in dichloromethane (3 mL), stirred at room temperature overnight, then adjusted with 1N NaOH solution to pH=12, stirred continuously for 20 min, and the reaction was monitored by LCMS. A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com