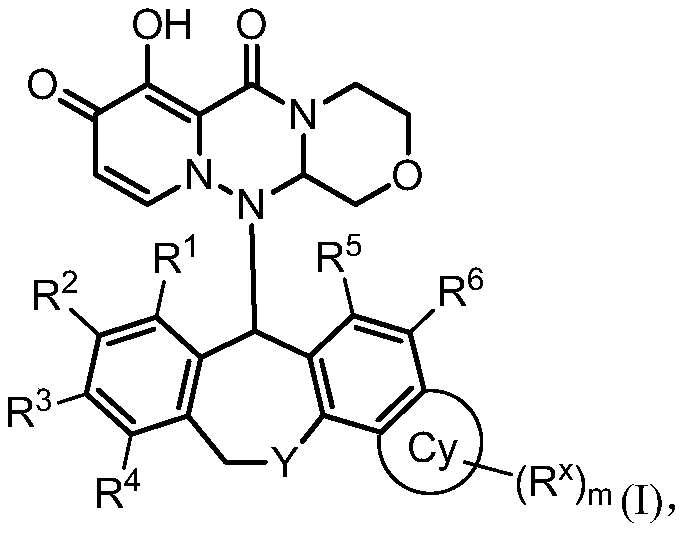
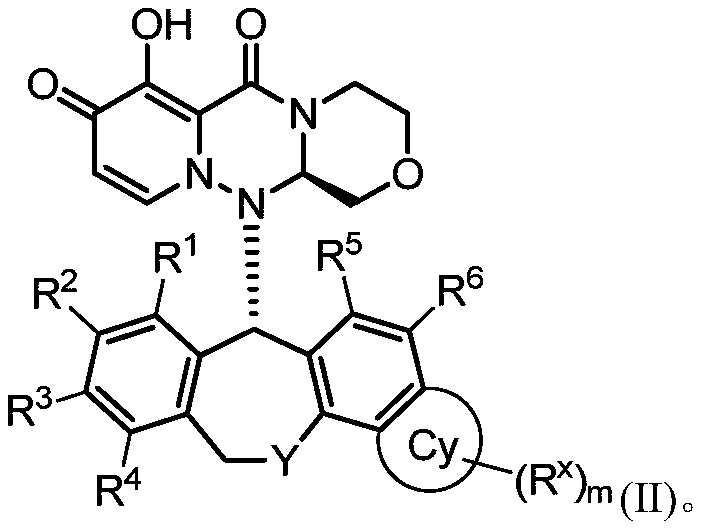
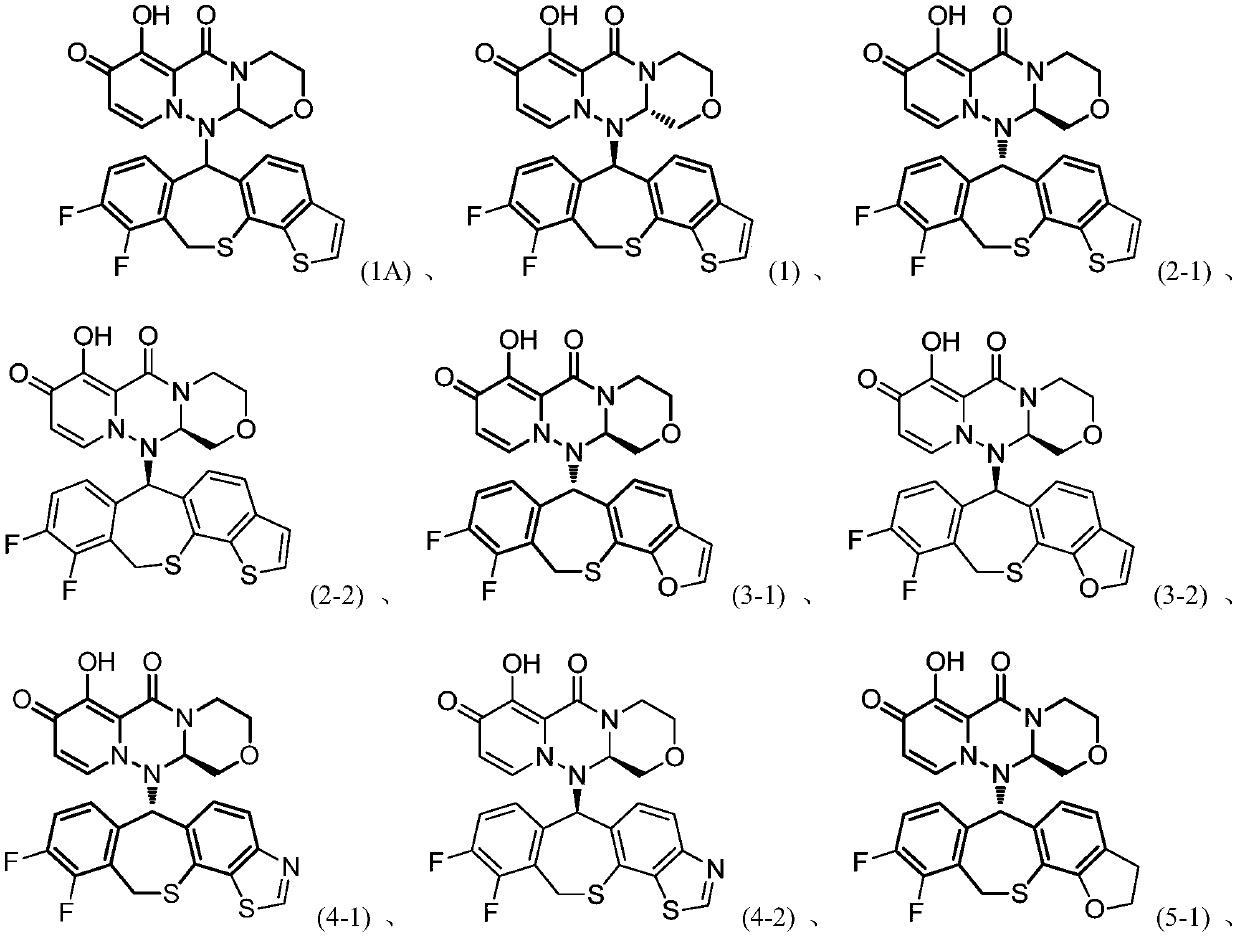
Influenza virus replication inhibitor and uses thereof
A technology of solvates and compounds, applied in antiviral agents, medical preparations containing active ingredients, organic chemistry, etc., to achieve excellent in vivo pharmacodynamic properties, good influenza virus, and good druggability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0286] Example 1: (R)-12-((S)-9,10-difluoro-6,11-dihydrobenzo[e]thieno[3',2':5,6]benzo[1 ,2-b]thiepan-6-yl)-7-hydroxyl-3,4,12,12a-tetrahydro-1H-[1,4]oxazino[3,4-c]pyrido[ 2,1-f][1,2,4]triazine-6,8-dione and (S)-12-((R)-9,10-difluoro-6,11-dihydrobenzo[e ]thieno[3',2':5,6]benzo[1,2-b]thiepan-6-yl)-7-hydroxy-3,4,12,12a-tetrahydro-1H- Mixture of [1,4]oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazine-6,8dione Mixture:
[0287]
[0288] Step 1): 7-(Benzyloxy)-3,4,12,12a-tetrahydro-1H-[1,4]oxazino[3,4-c]pyrido[2,1-f] Synthesis of [1,2,4]triazine-6-,8-dione
[0289]
[0290] The title compound was prepared by referring to the synthesis method disclosed in the patent application WO 2016175224.
[0291] Step 2): Synthesis of benzo[b]thiophene-7-thiol
[0292] 7-Bromobenzothiophene (5.00g, 23.5mmol) was added into THF (50mL), cooled to -78°C, sulfur powder (0.79g, 25mmol) was added, and stirred at this temperature for 0.5 hours. A tetrahydrofuran solution of tert-butyllithium (3...
Embodiment 2
[0315] Example 2 (R)-12-((S)-9,10-difluoro-6,11-dihydrobenzo[e]thieno[3',2':5,6]benzo[1, 2-b]thiepan-6-yl)-7-hydroxy-3,4,12,12a-tetrahydro-1H-[1,4]oxazino[3,4-c]pyrido[2 ,1-f][1,2,4]triazine-6,8 dione (2-1) and (R)-12-((R)-9,10-difluoro-6,11-dihydro Benzo[e]thieno[3',2':5,6]benzo[1,2-b]thiepan-6-yl)-7-hydroxy-3,4,12,12a-tetra Hydrogen-1H-[1,4]oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazine-6,8-dione (2-2)
[0316]
[0317] Step 1): (R)-7-(Benzyloxy)-3,4,12,12a-tetrahydro-1H-[1,4]oxazino[3,4-c]pyrido[2, Synthesis of 1-f][1,2,4]triazine-6,8-dione
[0318]
[0319] The title compound was prepared by referring to the synthesis method disclosed in the patent application WO 2017221869.
[0320] Step 2): (12aR)-7-(benzyloxy)-12-(9,10-difluoro-6,11-dihydrobenzo[e]thieno[3', 2':5,6]benzo[1,2-b]thiepan-6-yl)-3,4,12,12a-tetrahydro-1H-[1,4]oxazino[3, 4-c]pyrido Synthesis of [2,1-f][1,2,4]triazine-6,8-dione
[0321] 9,10-difluoro-6,11-dihydrobenzo[e]thieno[3',2':5,6]benzo[1...
Embodiment 3
[0331] Example 3 (R)-12-((S)-9,10-difluoro-6,11-dihydrobenzo[5,6]thiepano[3,2-g]benzofuran- 6-yl)-7-hydroxy-3,4,12,12a-tetrahydro-1H-[1,4]oxazino[3,4-c]pyrido[2,1-f][1,2 ,4] Triazine-6,8-dione (3-1) and (R)-12-((R)-9,10-difluoro-6,11-dihydrobenzo[5,6]sulfur Heptacyclo[3,2-g]benzofuran-6-yl)-7-hydroxy-3,4,12,12a-tetrahydro-1H-[1,4]oxazino[3,4- c]pyrido[2,1-f][1,2,4]triazine-6,8-dione (3-2)
[0332]
[0333] Step 1): Synthesis of Benzofuran-7-thiol
[0334] Dissolve 7-bromobenzofuran (7.00g, 35.50mmol), sulfur (1.19g, 37.20mmol) in THF (5mL), under nitrogen protection, stir at -78°C for 20 minutes, then add tert-butyllithium (55.00 mL, 72.00 mmol, 1.30 mol / L) was slowly added dropwise to the above reaction solution, and the reaction was continued at this temperature for 1 hour. Stop the reaction, add the reaction solution into saturated ammonium chloride solution (50mL), extract with diethyl ether (50mL×2), combine the organic phase, extract the obtained organic phase w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com