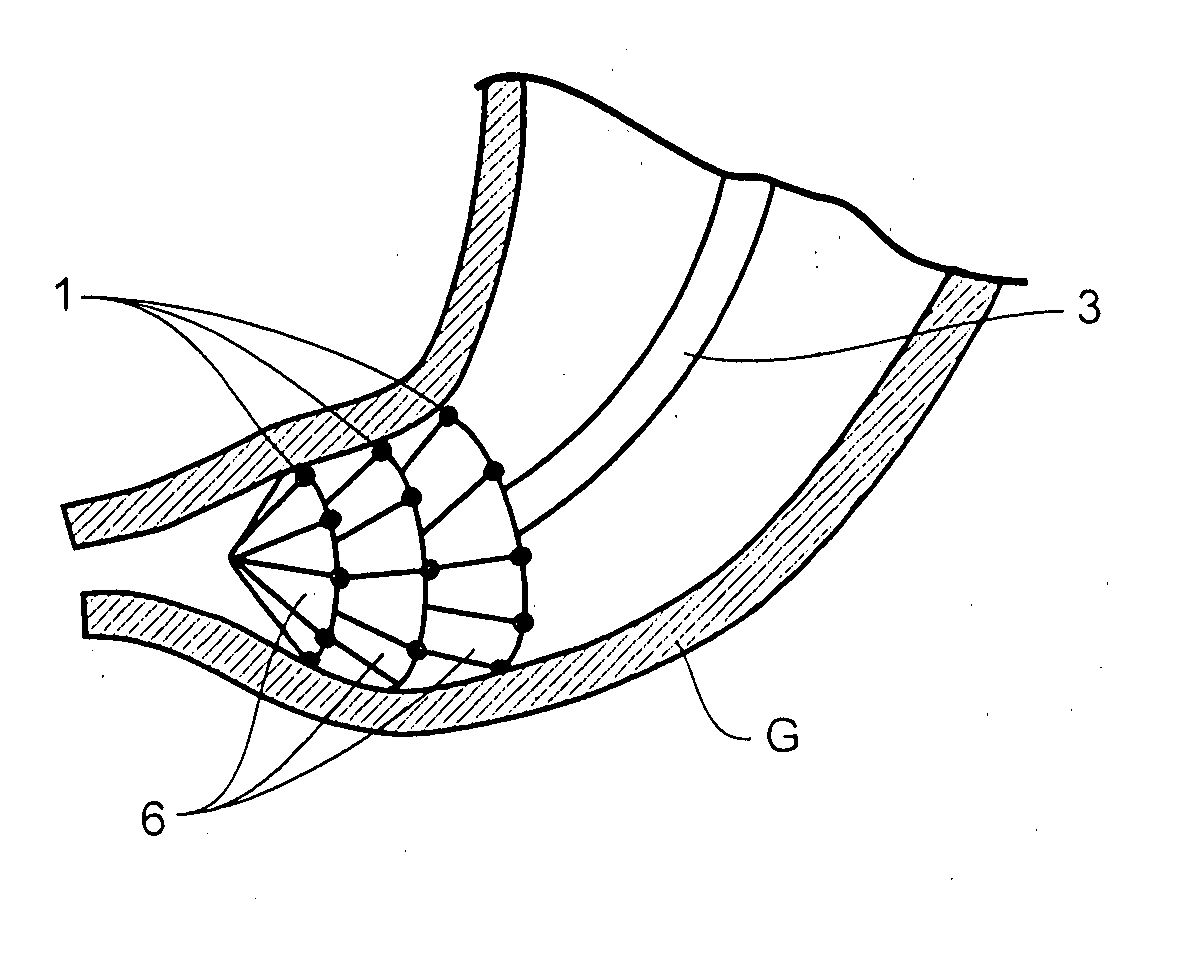
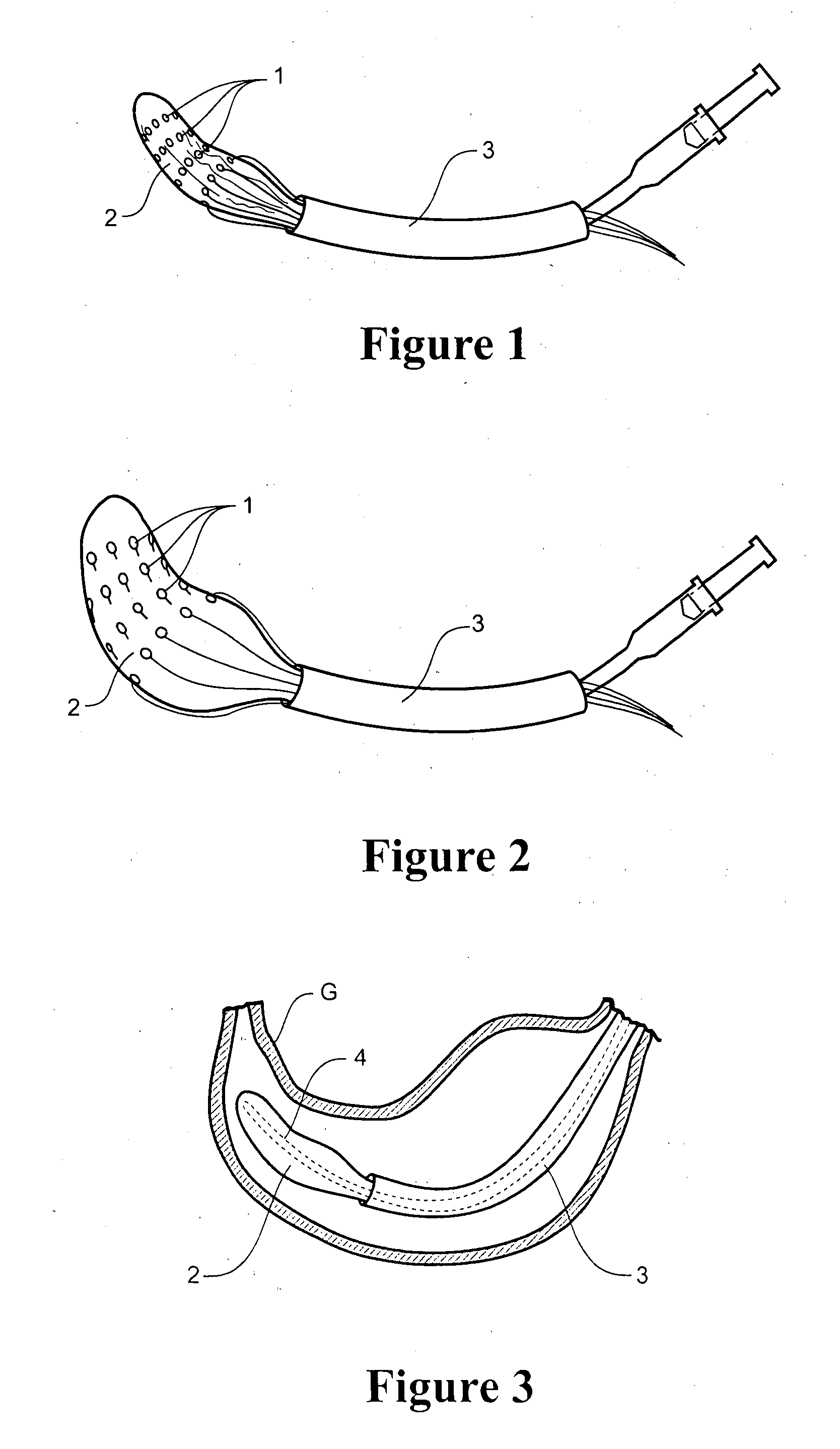
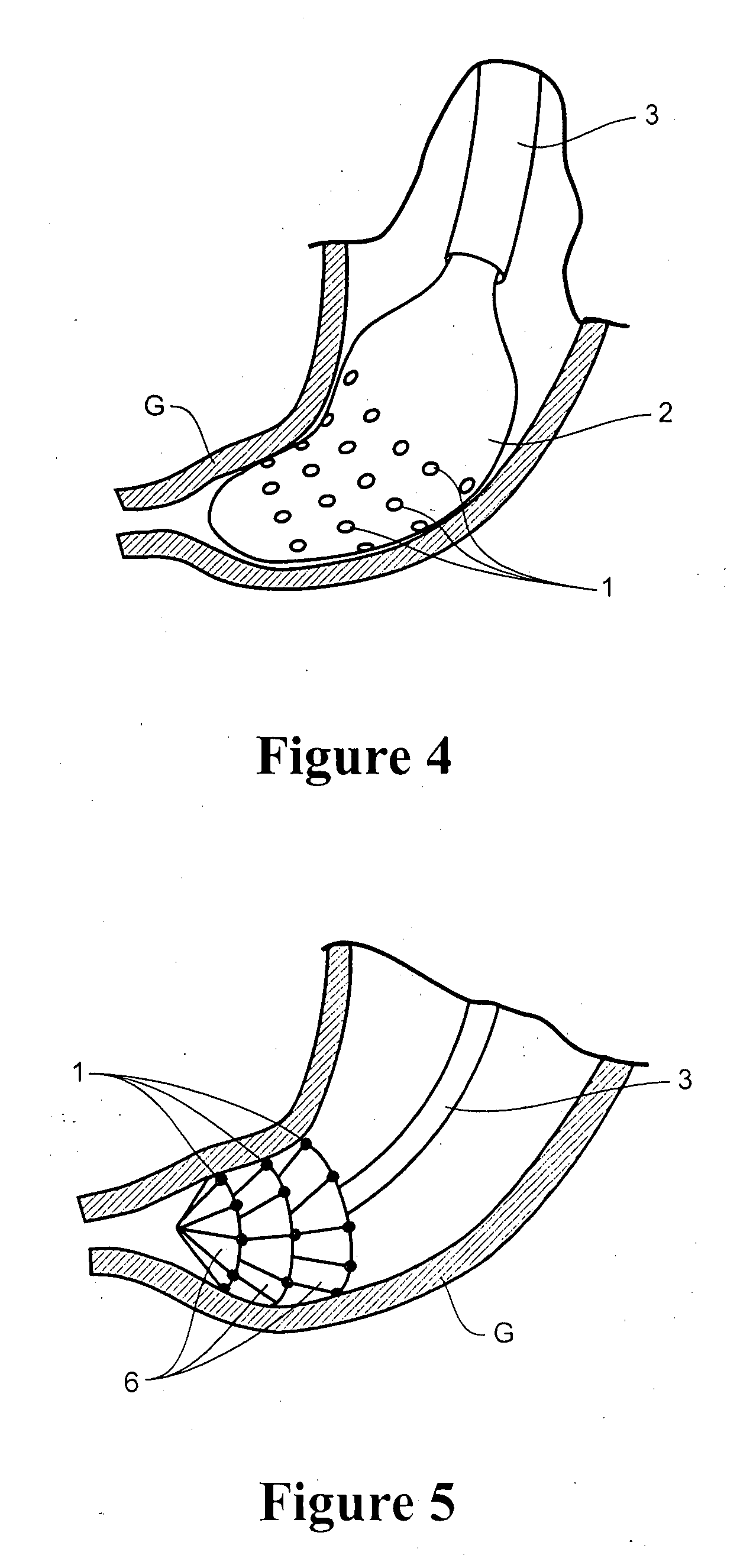
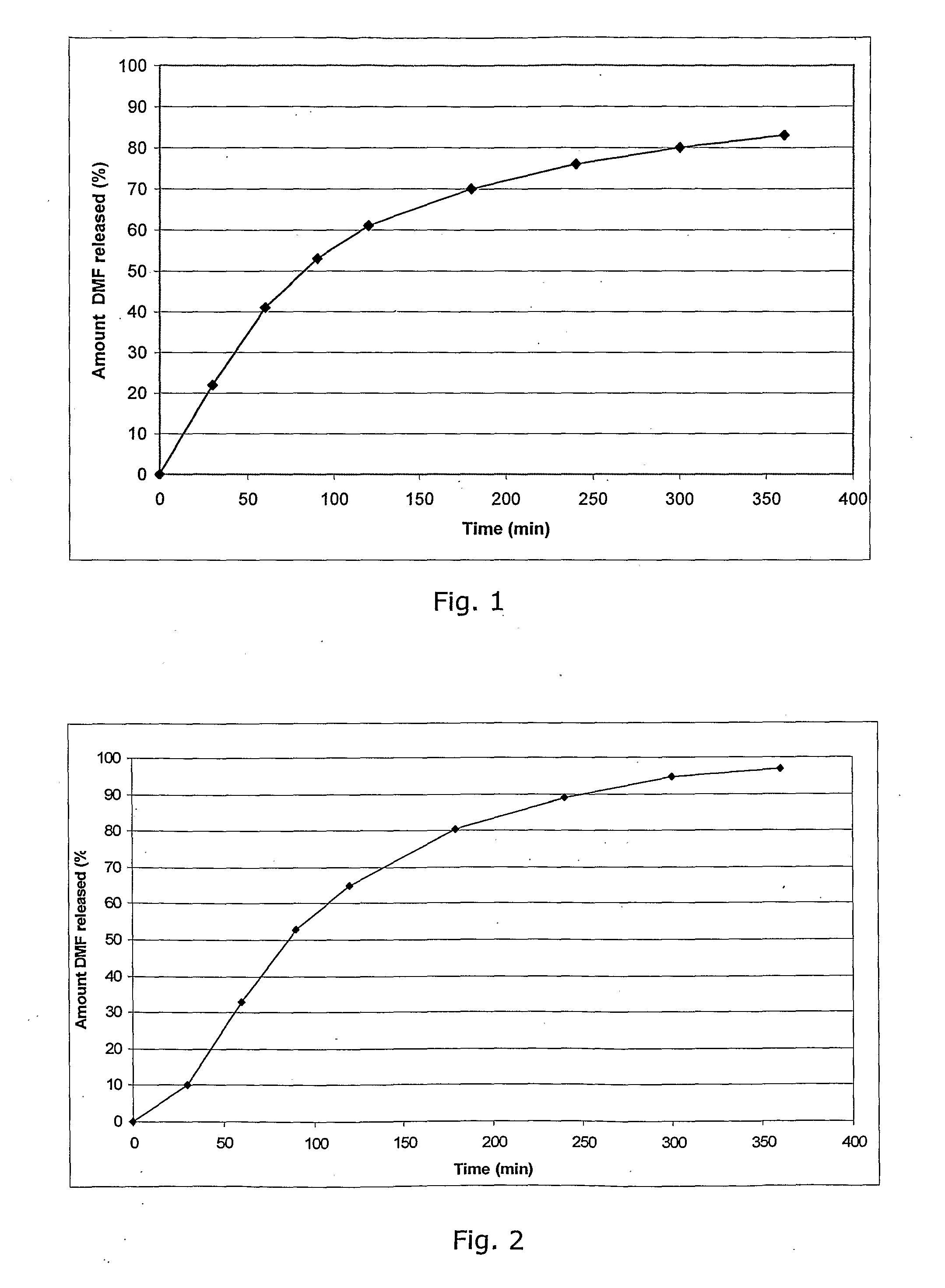
Patents
Literature
1420 results about "Gastro intestinal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gastrointestinal: Adjective referring collectively to the stomach and small and large intestines. The commonly used abbreviation for gastrointestinal is GI. (Outside of medicine, GI can also stand for galvanized iron, general issue or government issue - as in GI Joe).
Track for medical devices
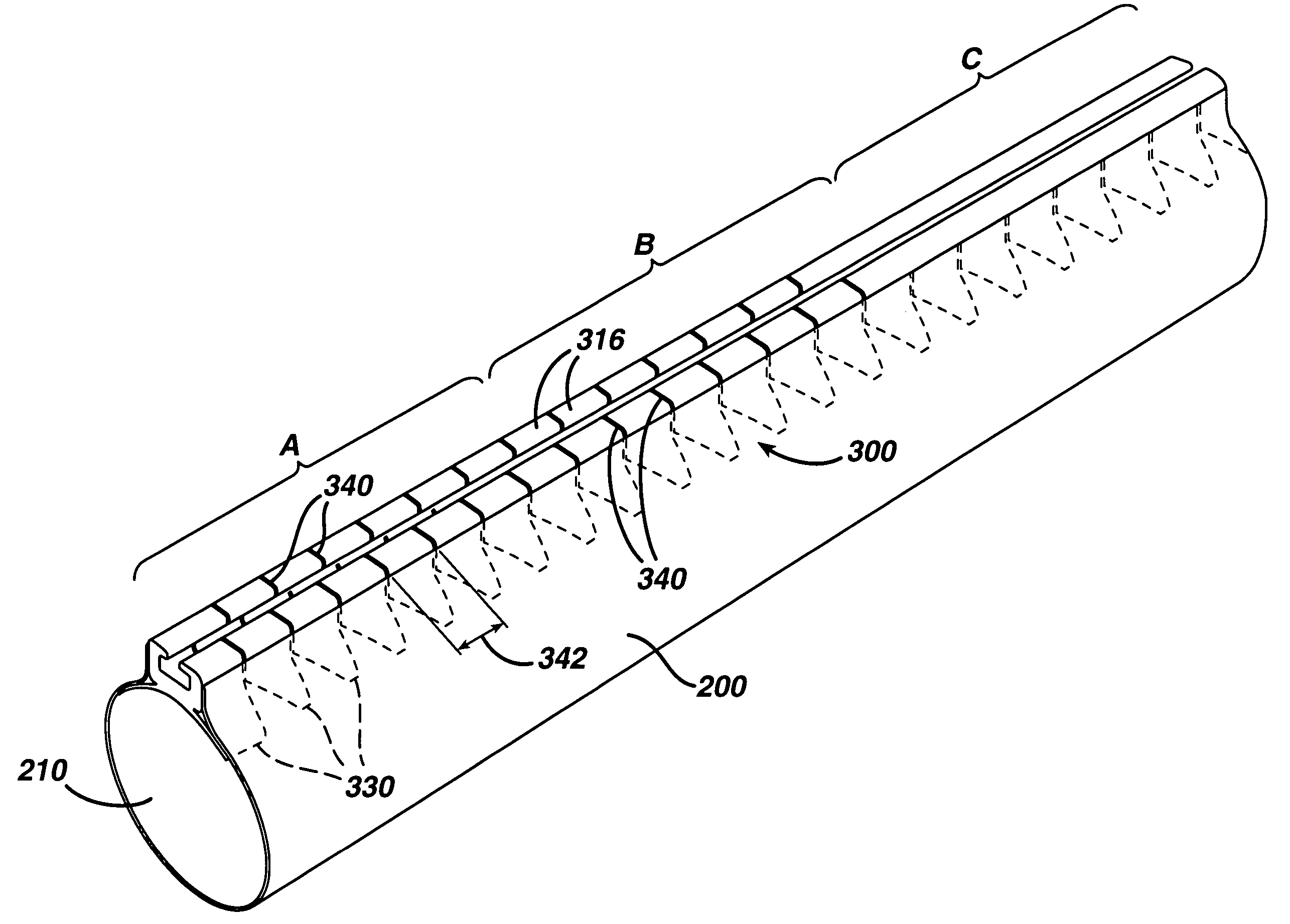
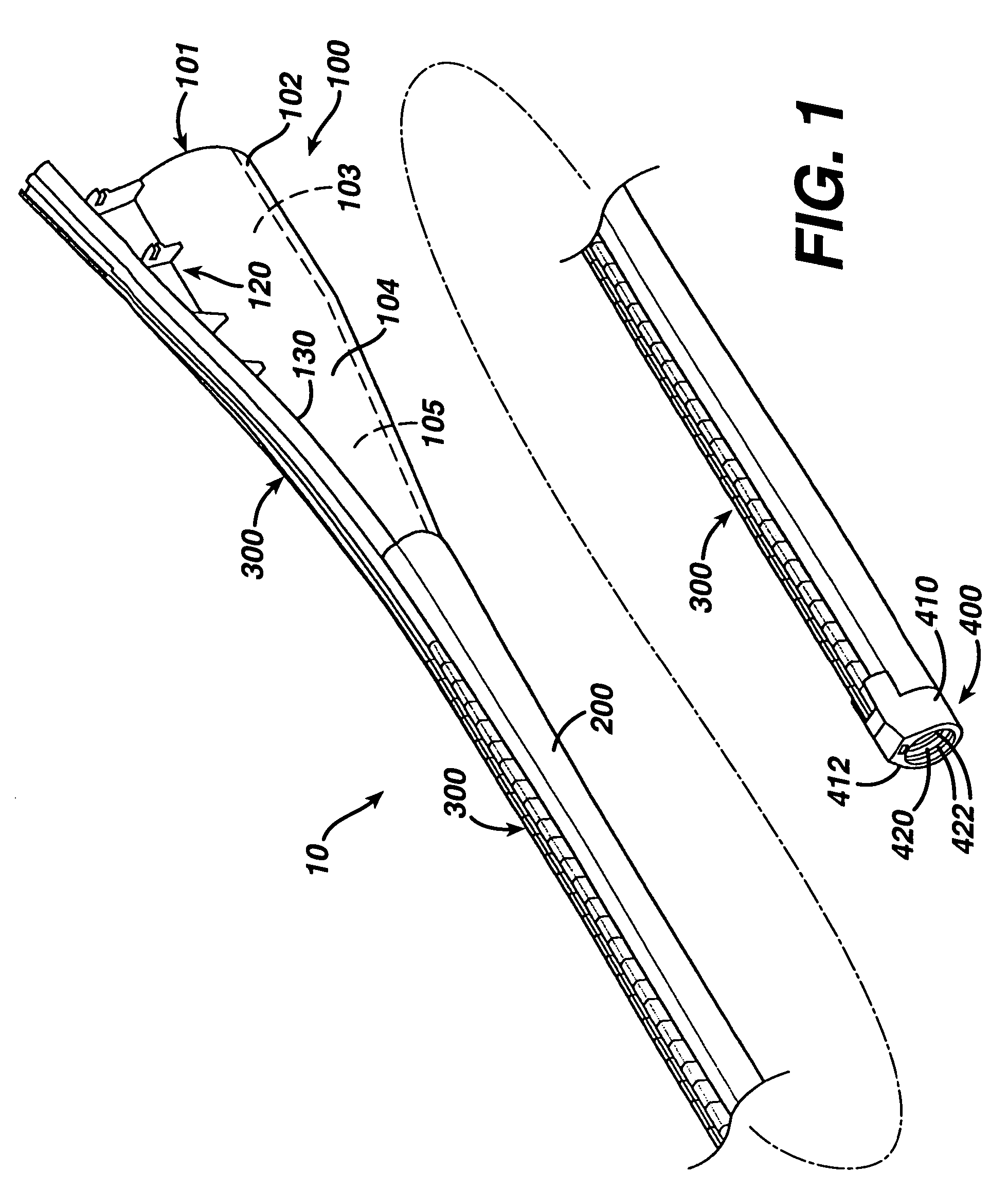
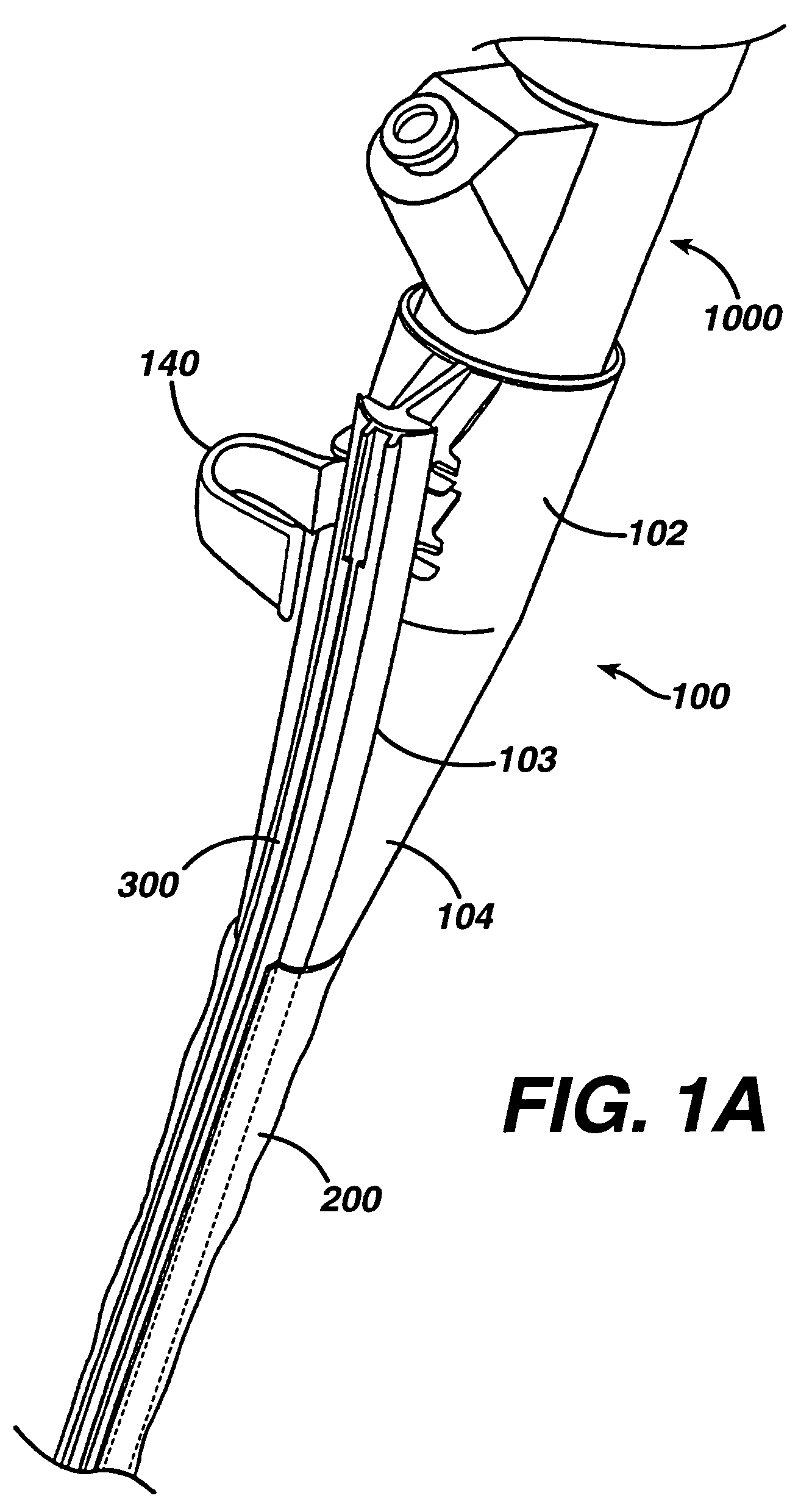
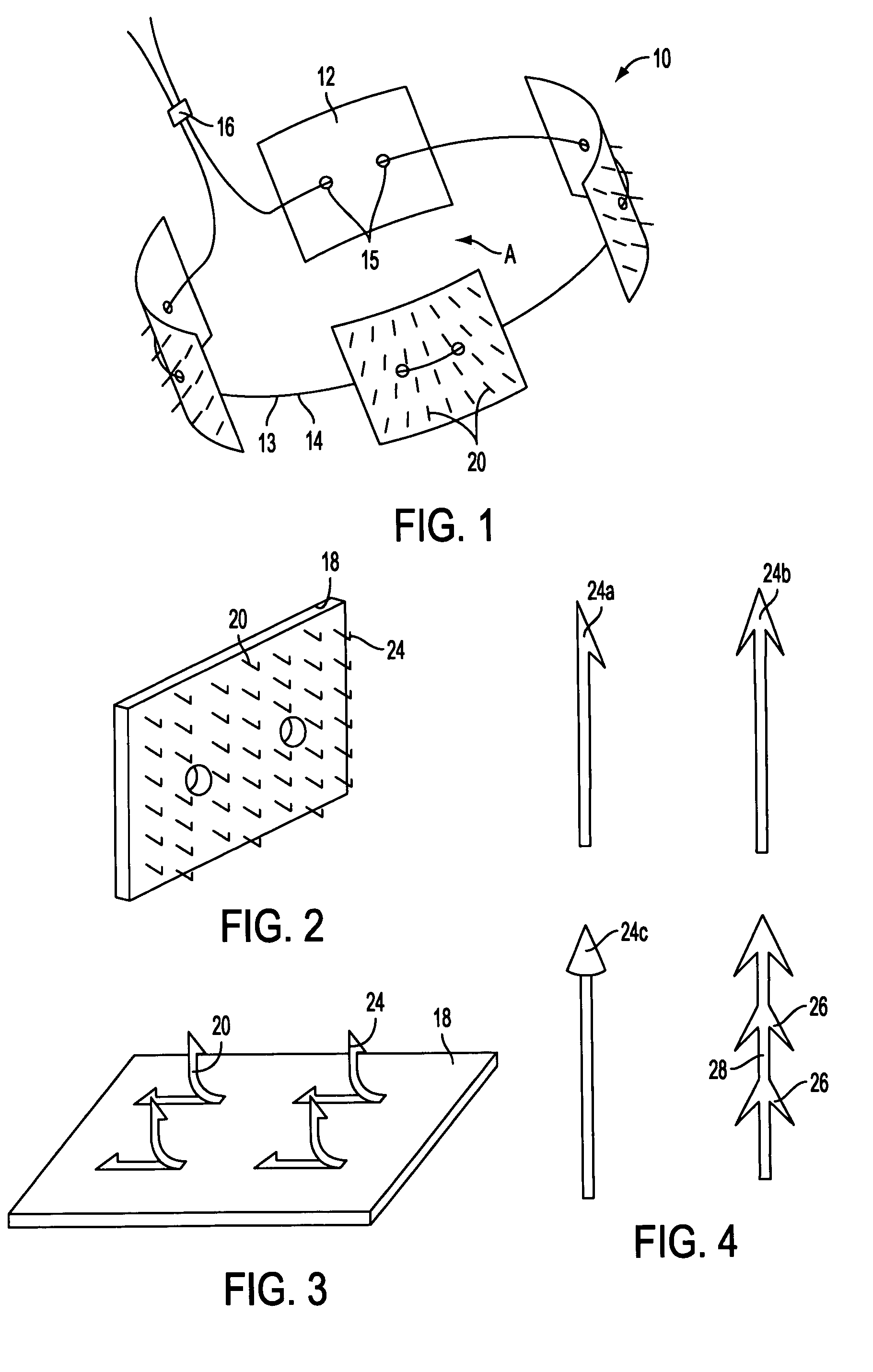
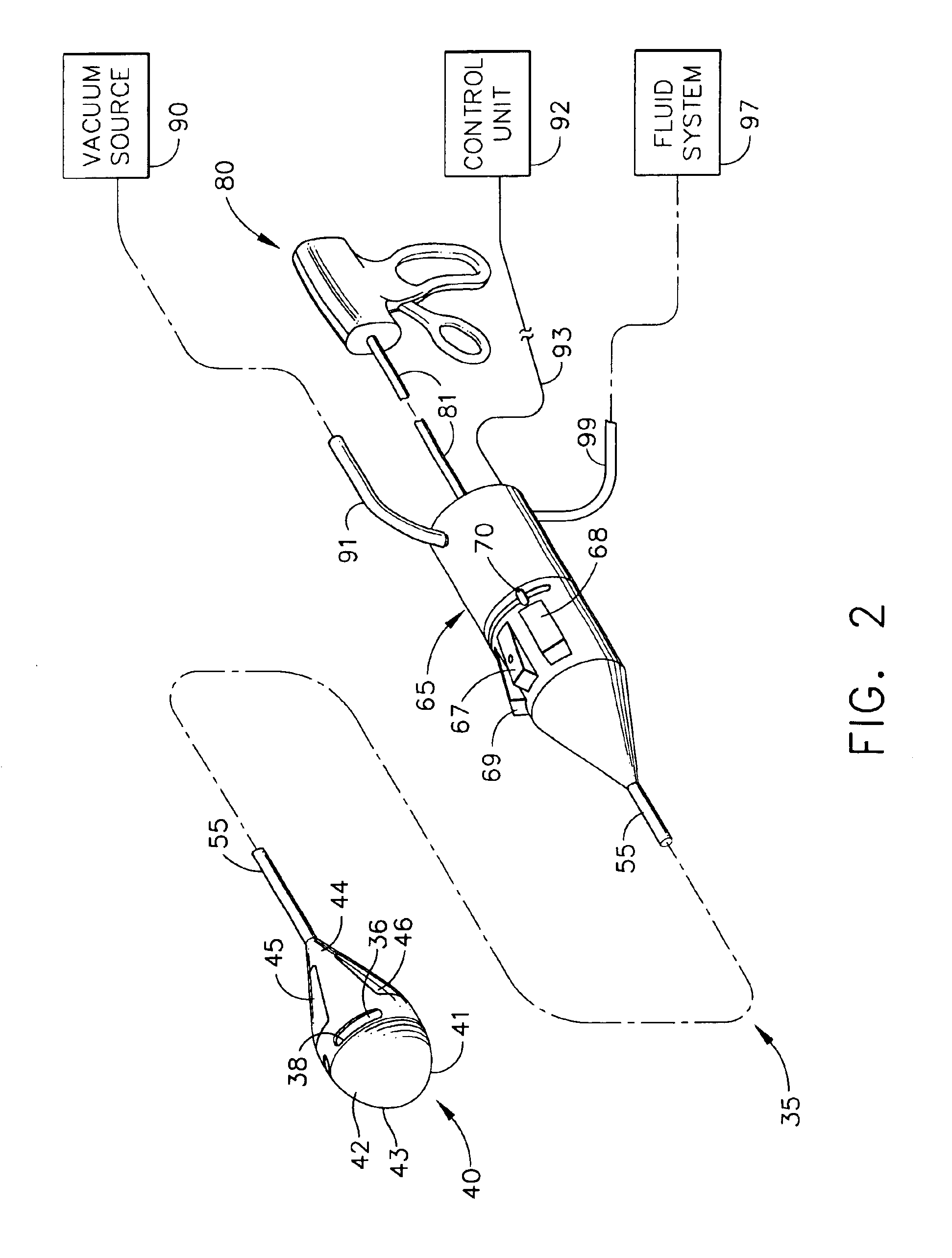
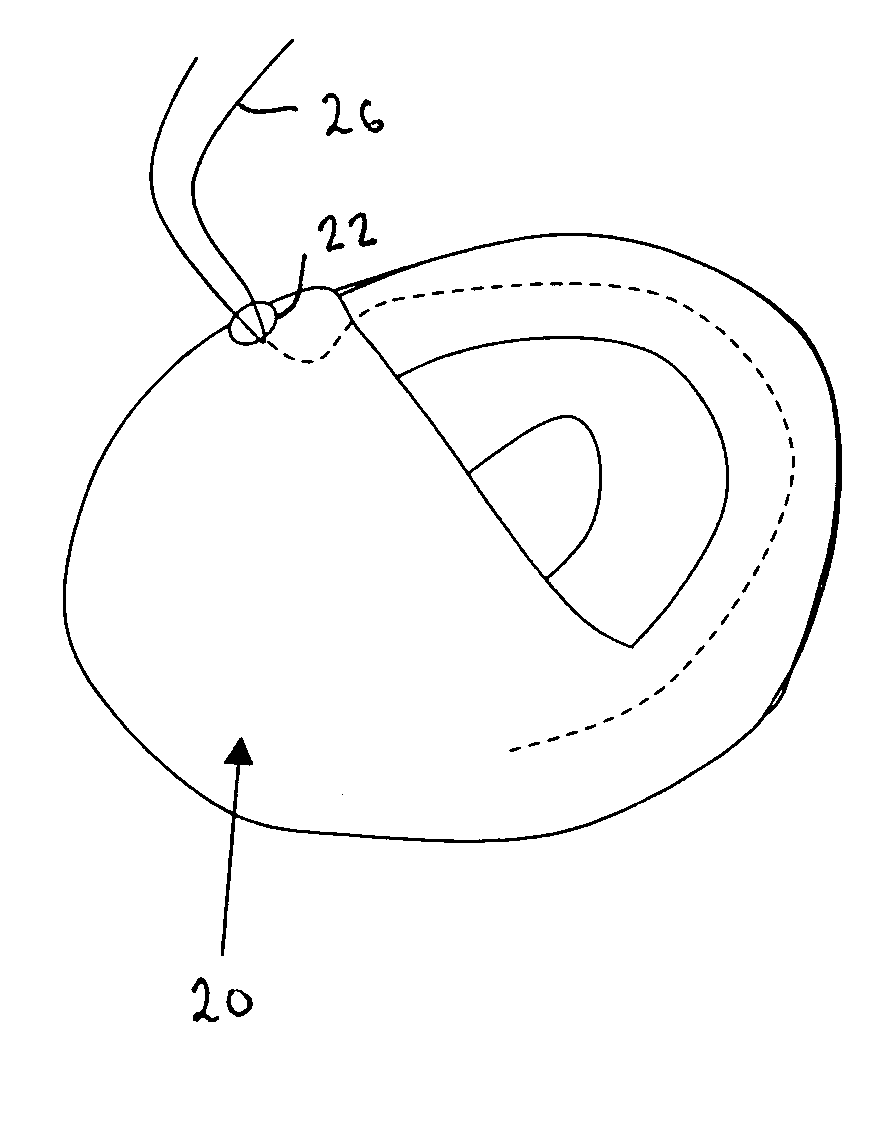
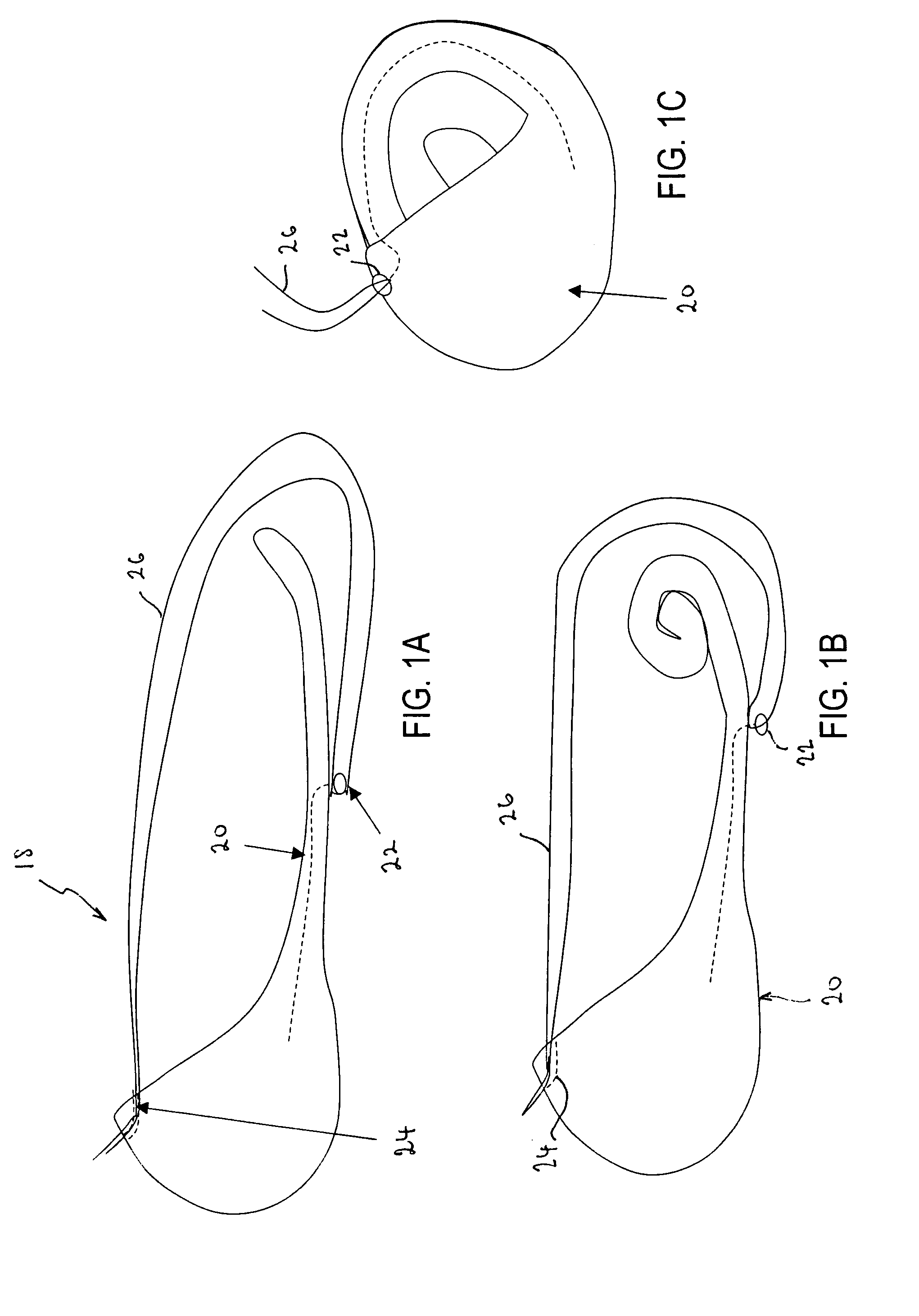
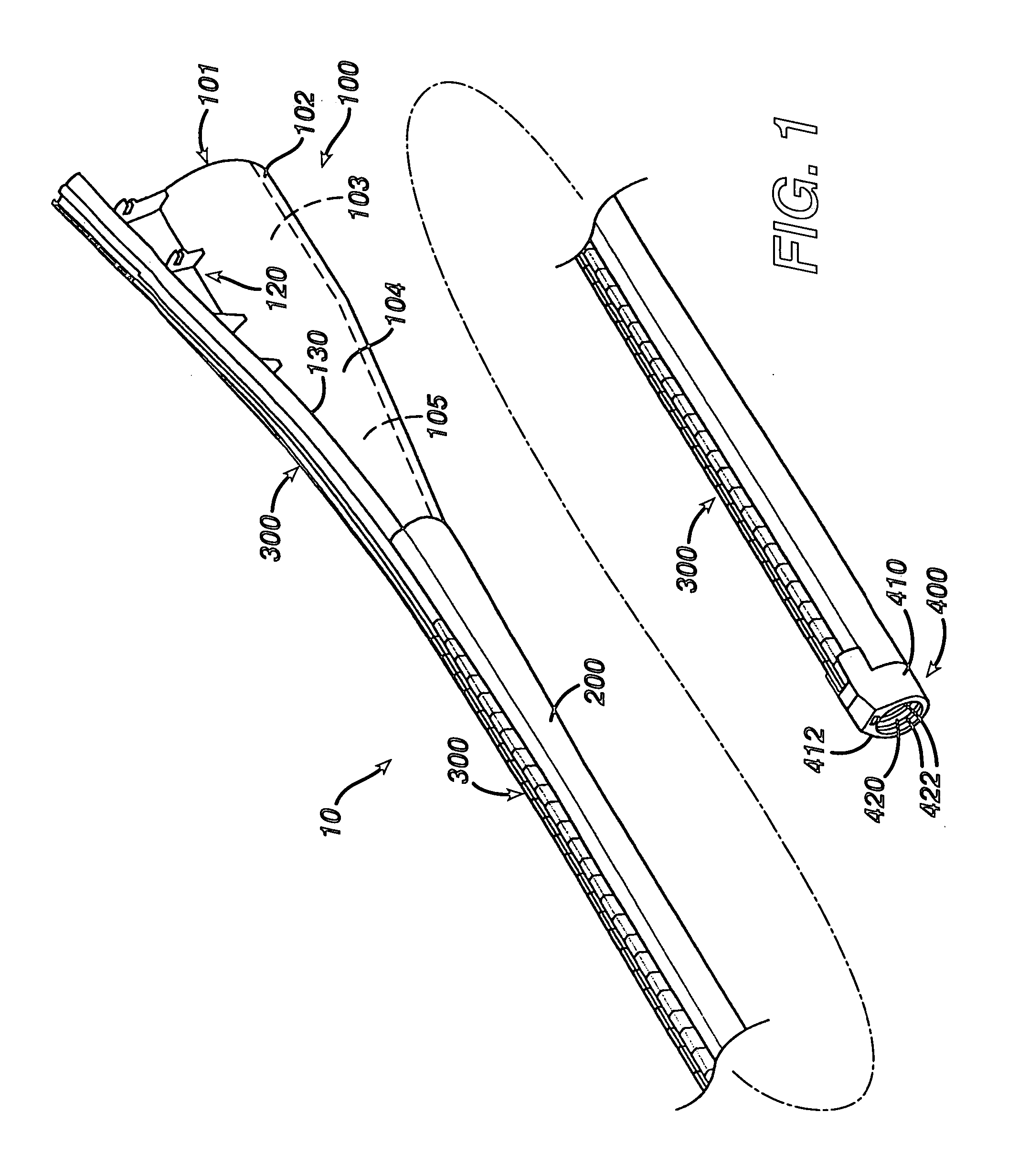
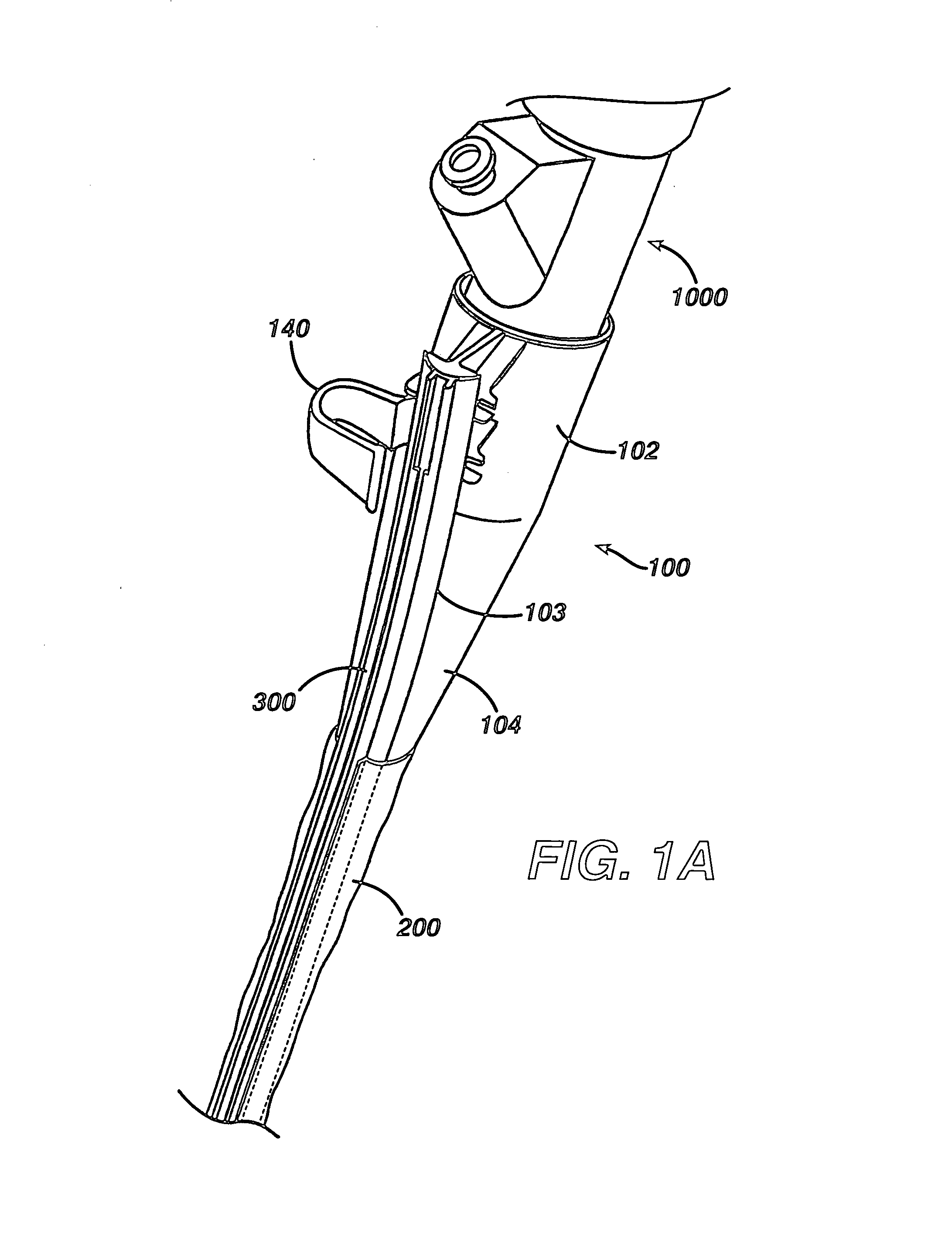
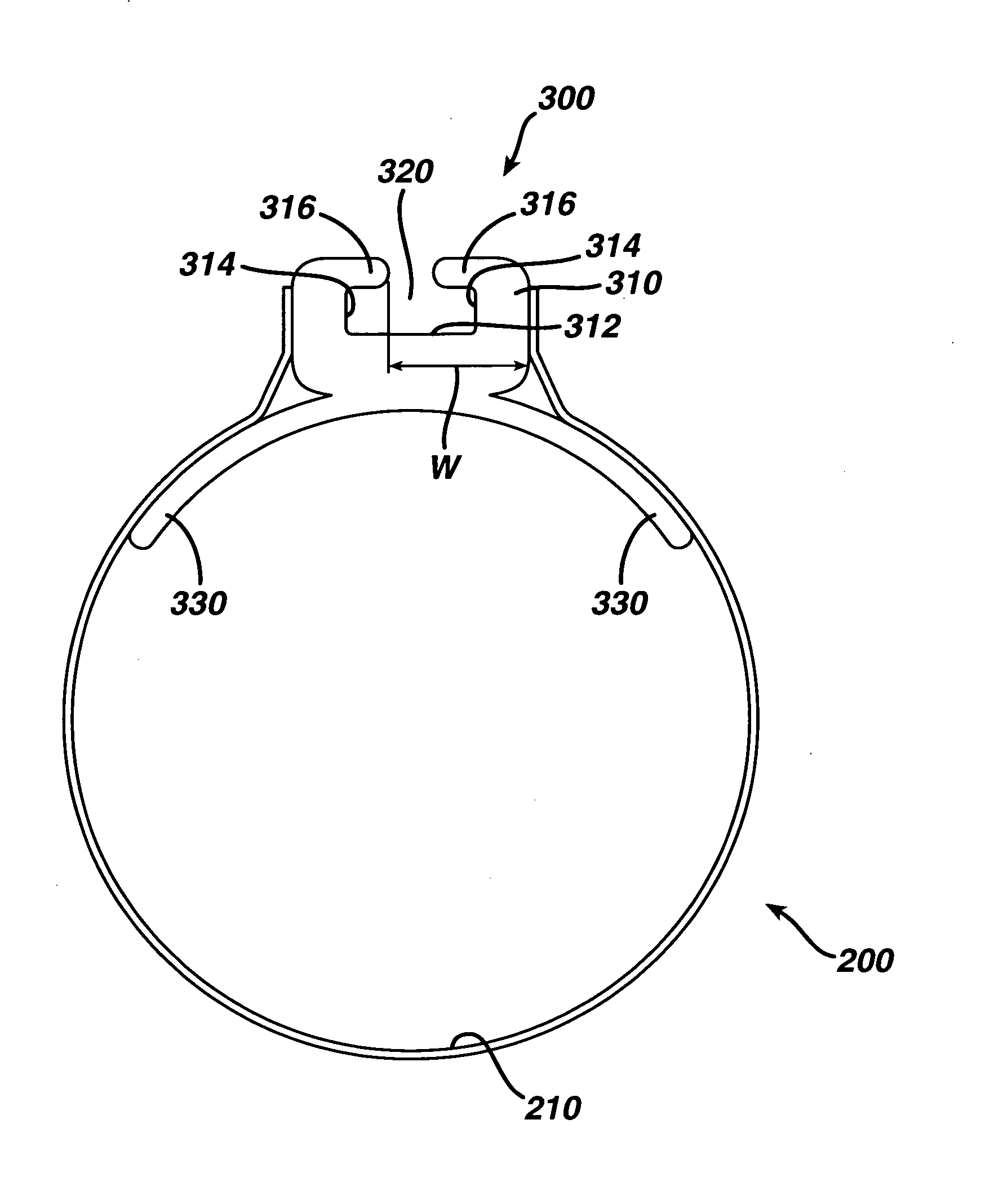
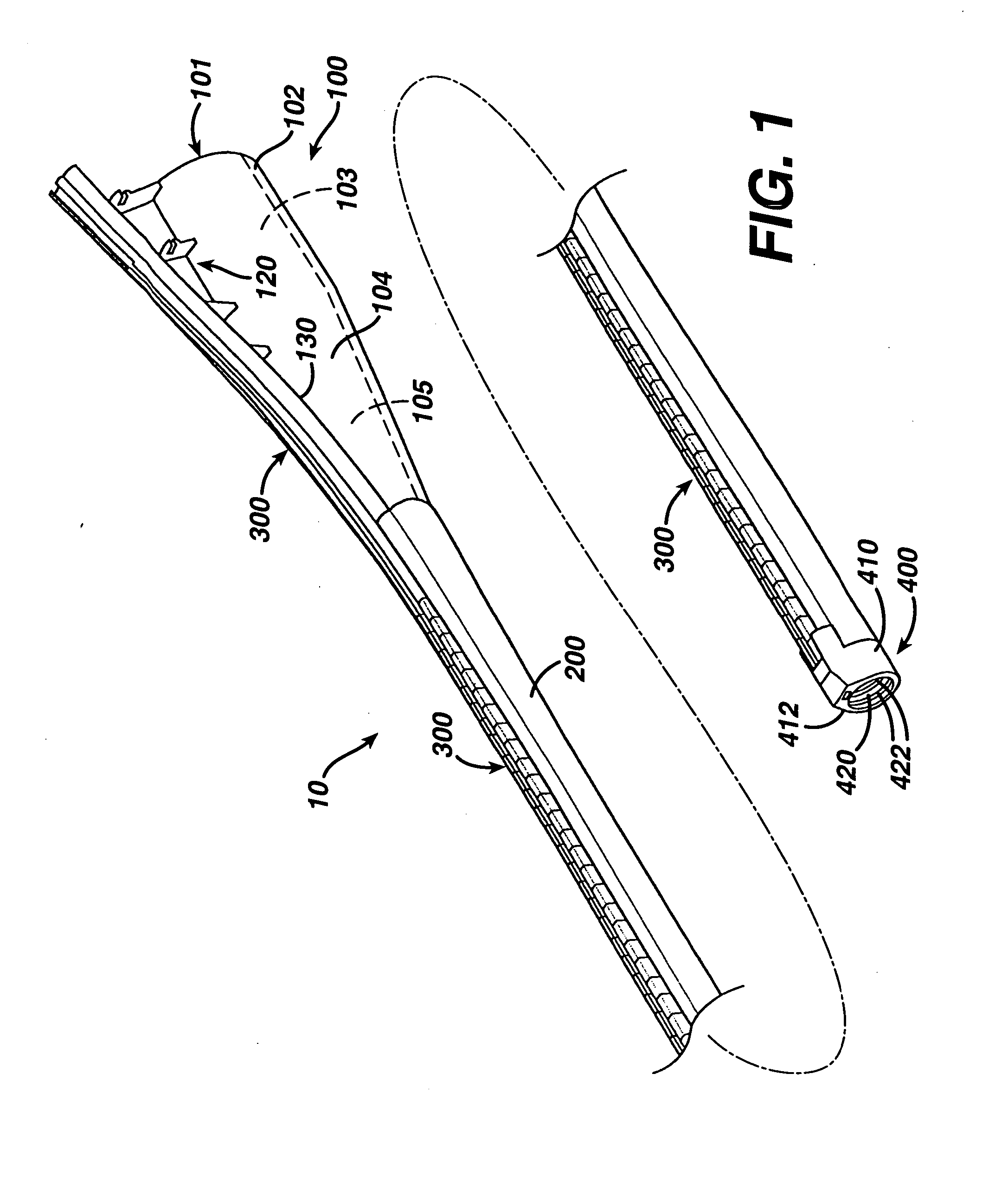
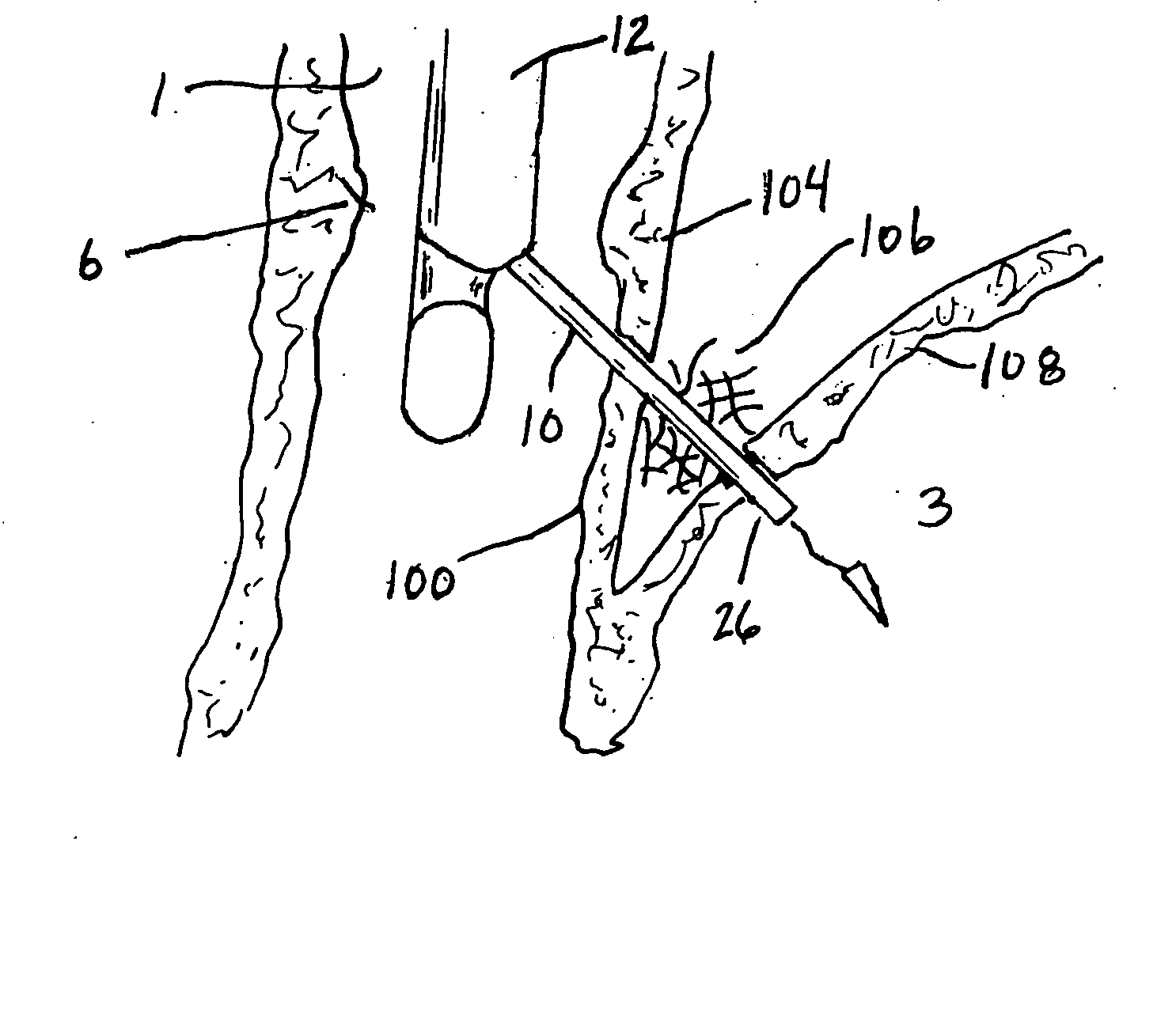
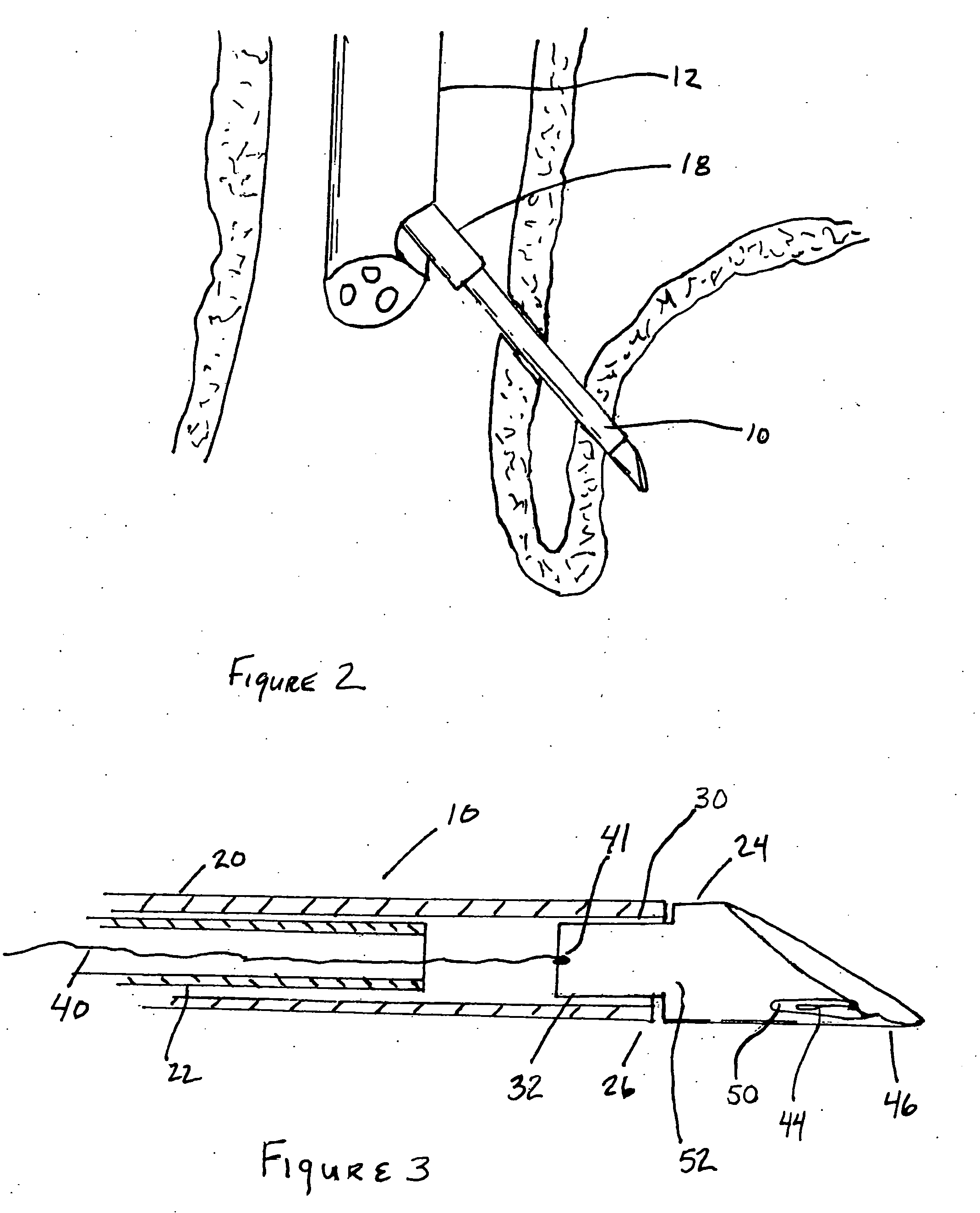
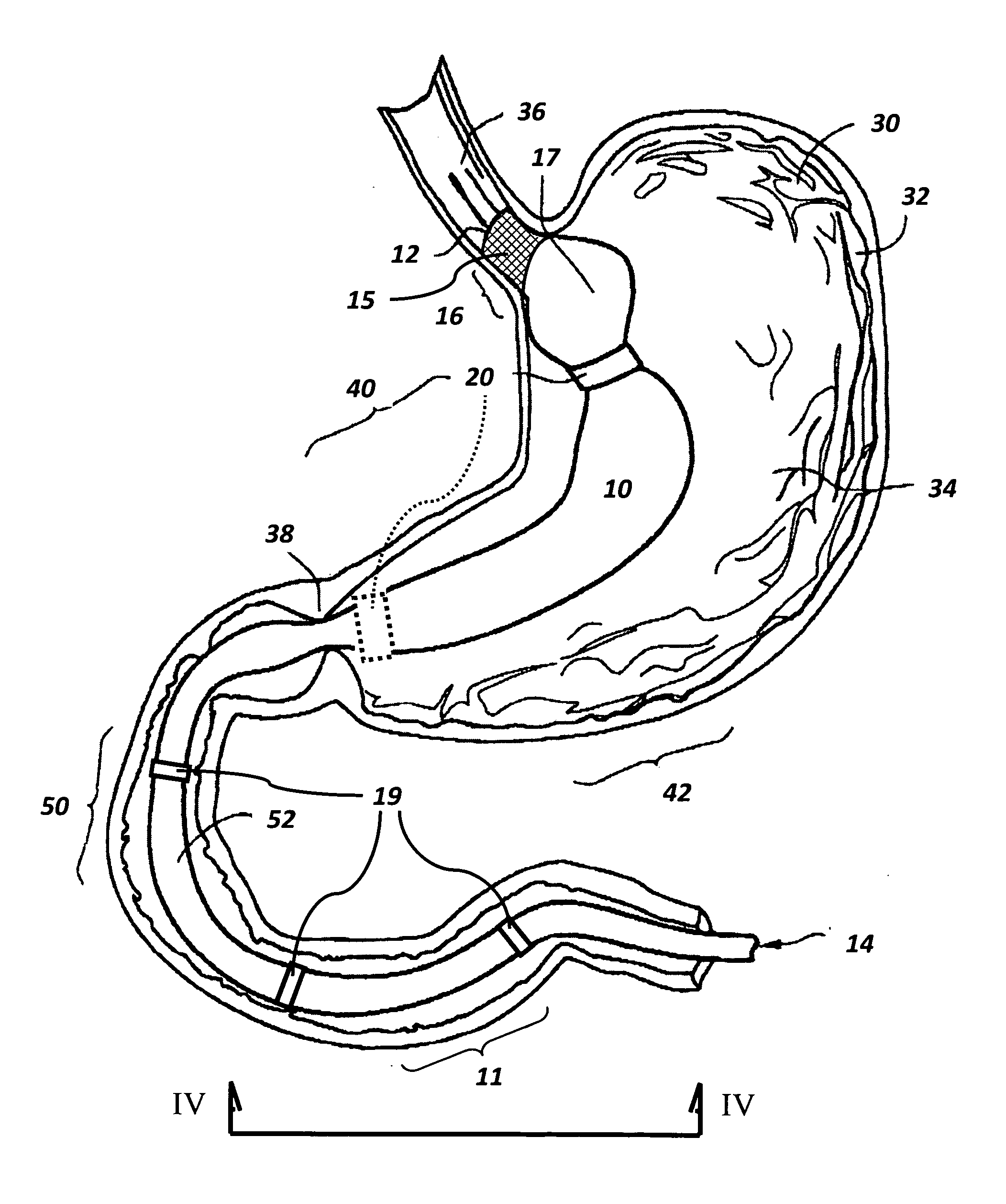
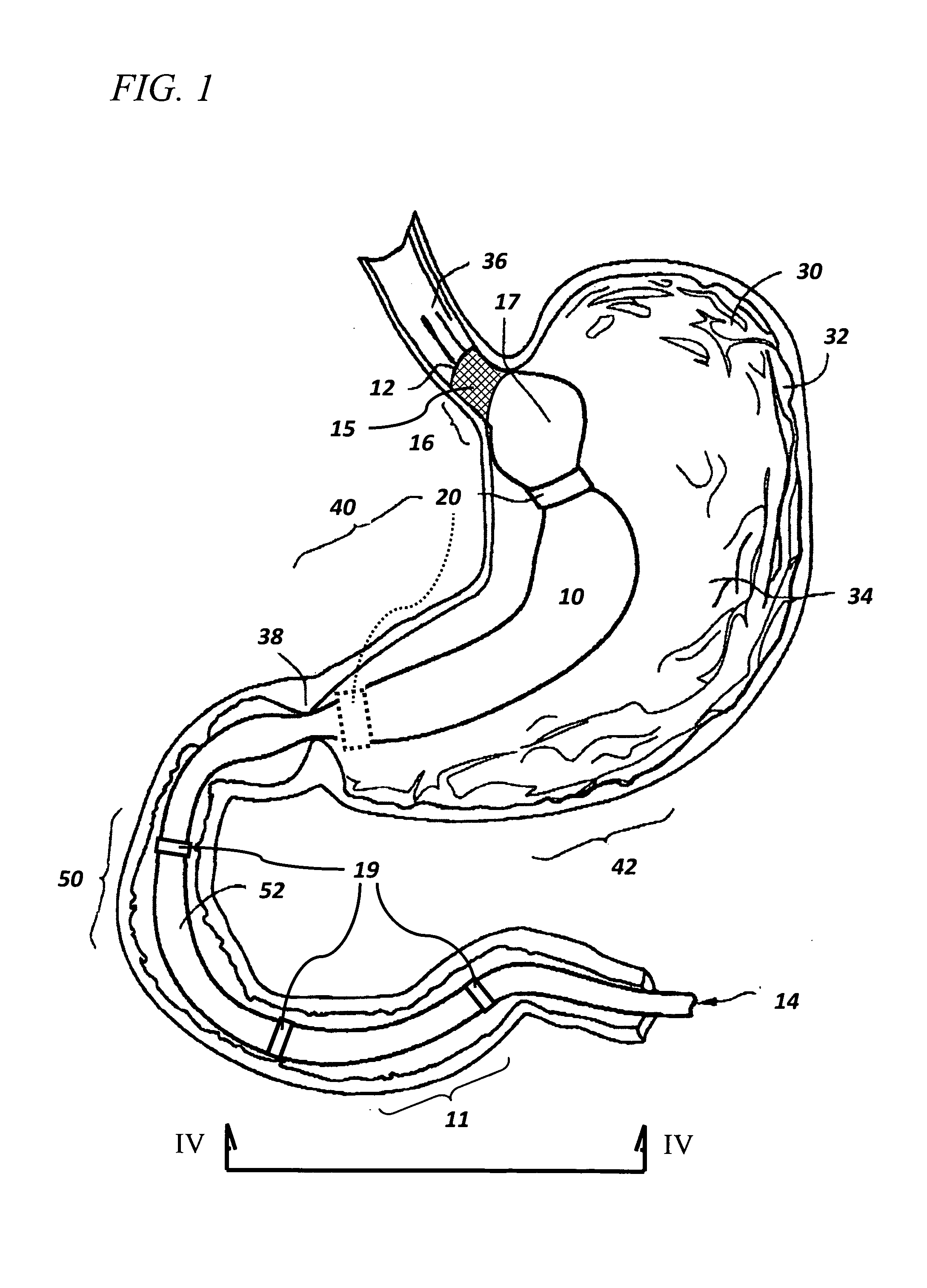
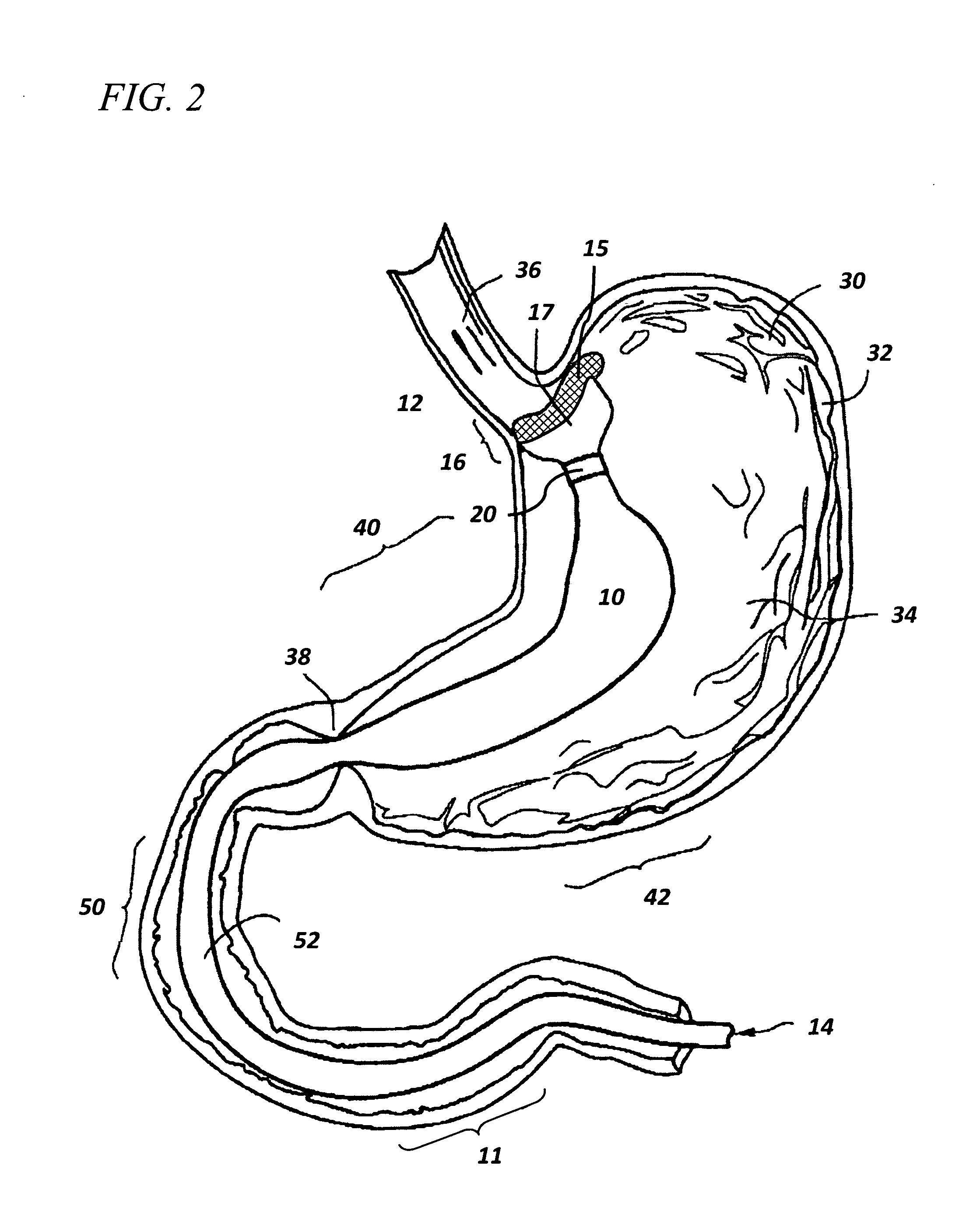
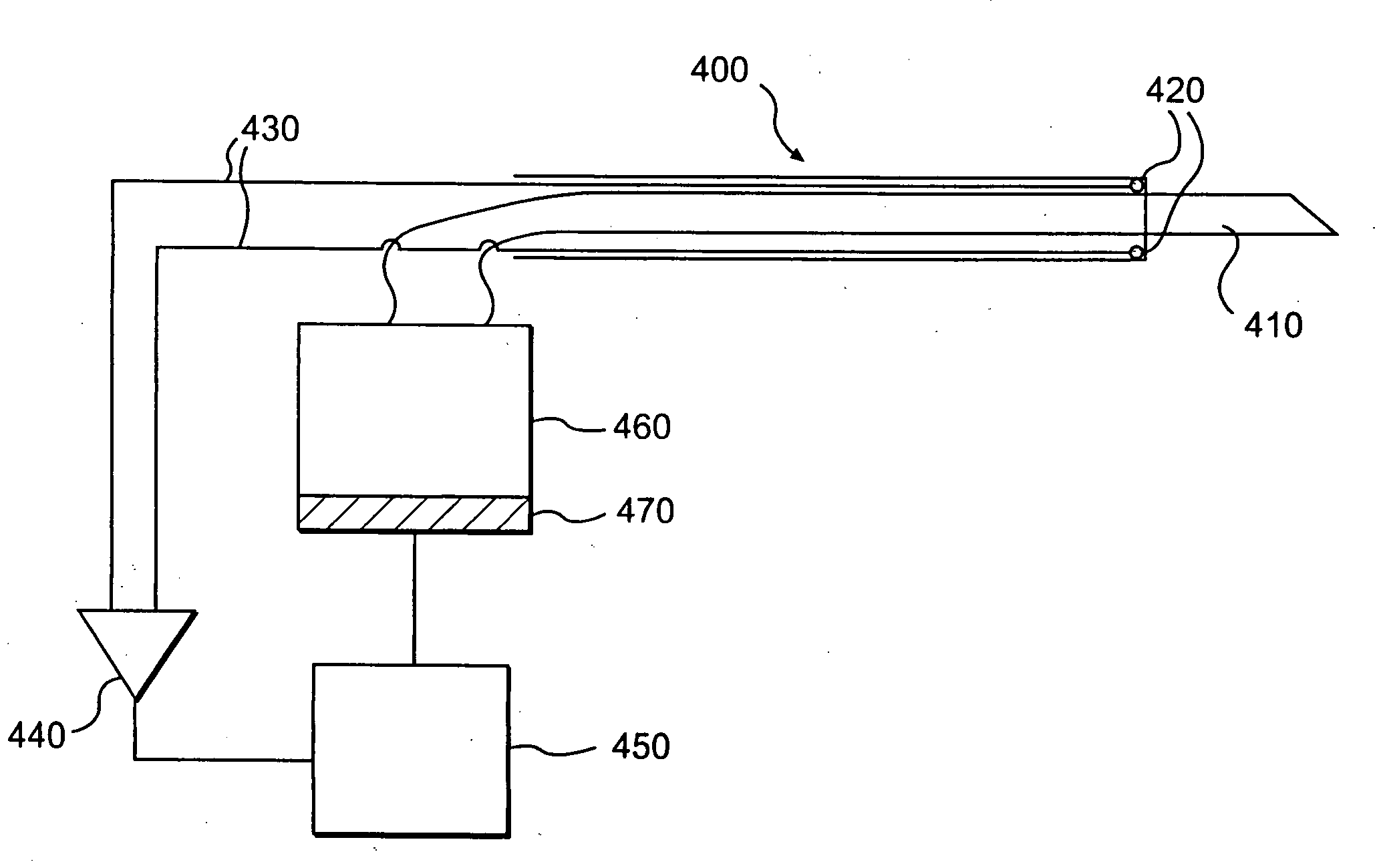
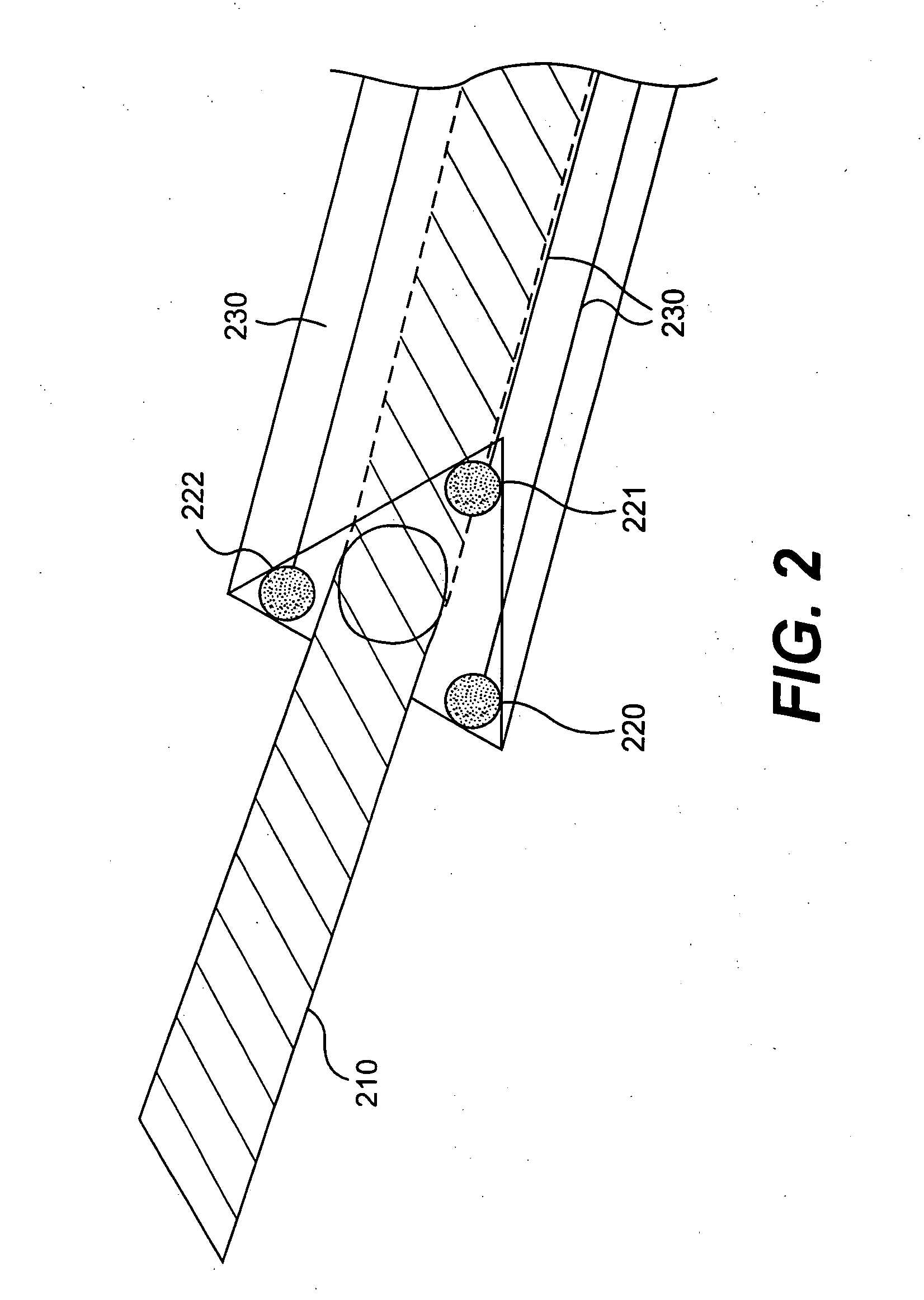
ActiveUS7615003B2Reduce in quantityQuickly and consistentlyGastroscopesCannulasMedical deviceFeeding tube
A medical apparatus and method useful for positioning one or more members within the gastro-intestinal tract is disclosed. The medical apparatus can include a track supported on a sheath sized to receive an endoscope, and a carrier slidable with respect to the track. A feeding tube accessory adapted to slidably engage the carrier is disclosed.
Owner:ETHICON ENDO SURGERY INC
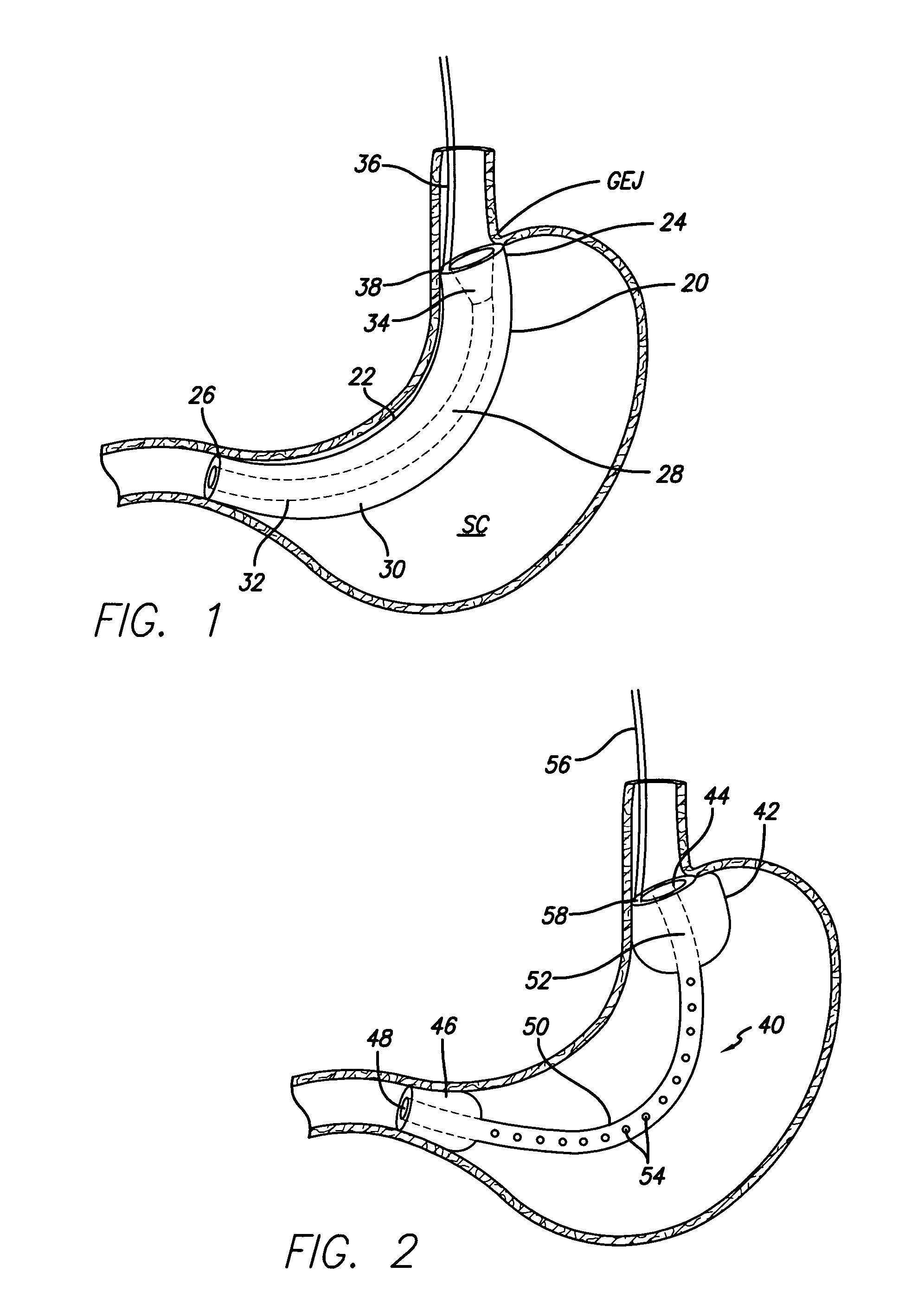
Implantable artificial partition and methods of use
InactiveUS7160312B2Reduce the cross-sectional areaReduce probabilitySuture equipmentsHeart valvesMedicineGastro intestinal
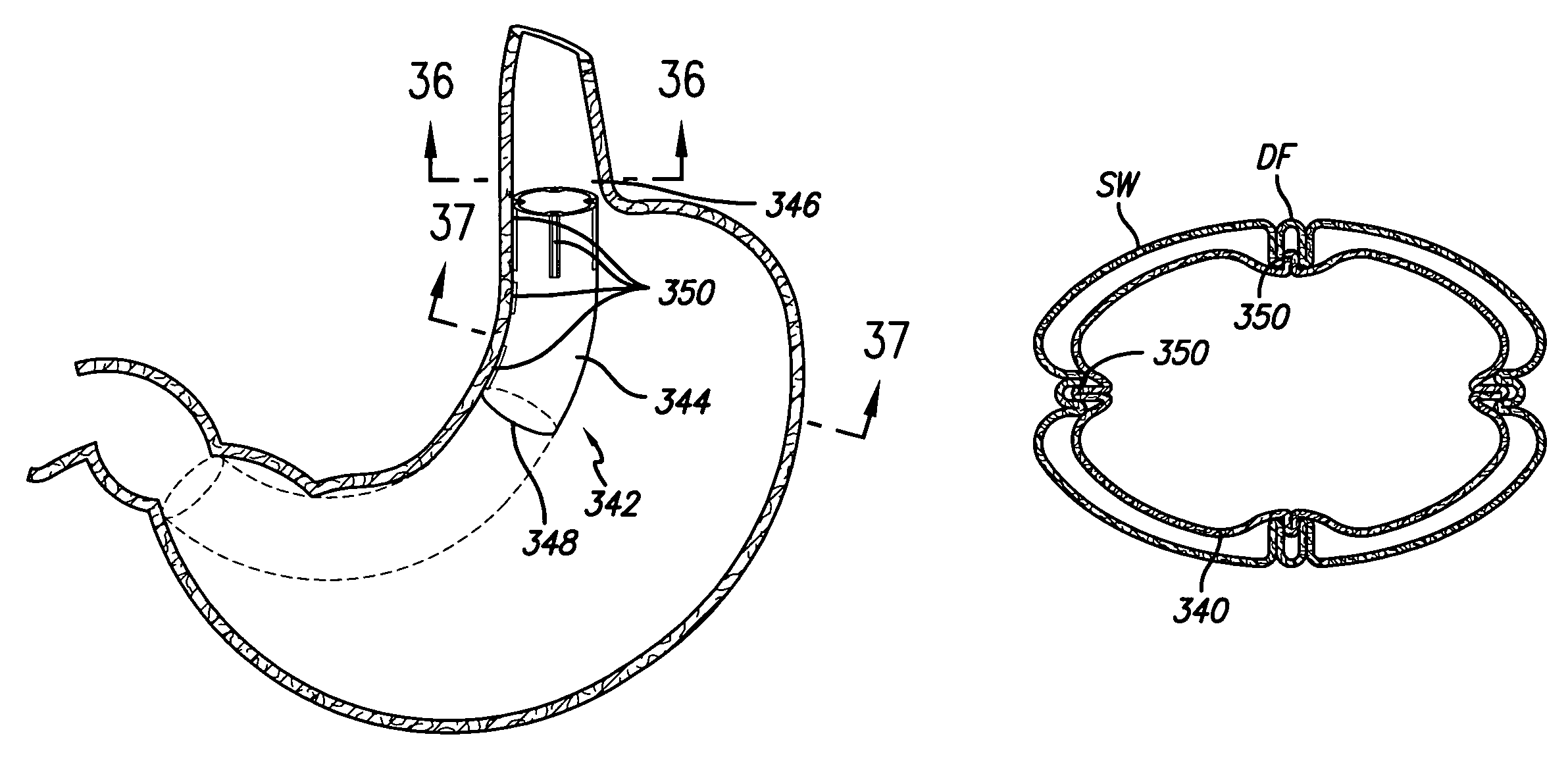
Apparatus and methods are provided for partitioning a gastro-intestinal lumen by intraluminally reducing a local cross-sectional area thereof. The apparatus comprises a plurality of anchors adapted for intraluminal penetration into a wall of the gastro-intestinal lumen to prevent migration or dislodgement of the apparatus, and a partition, which may include a drawstring or a toroidal balloon, coupled to the plurality of anchors to provide a local reduction in the cross-sectional area of the gastro-intestinal lumen.
Owner:USGI MEDICAL
Potentiation of immune responses with liposomal adjuvants
InactiveUS6090406AGood water solubilityPractical and convenientBacterial antigen ingredientsViral antigen ingredientsLipid formationOrganic acid
A high integrity liposome comprising at least one stabile lipid and at least one peptide-like therapeutic agent associated with said liposome, adapted for parenteral administration to an animal, including a human, and method according to manufacture and use. Immunizing dosage forms comprising a liposome and an immunogen, wherein said liposome and immunogen are present in an immunization dose. Additionally, a dosage form, including such form particularly adapted to producing an immune response, comprising a salt according to an organic acid derivative of a sterol and an immunogen wherein said organic acid derivative of a sterol and immunogen are present in an immunization dose, and method according to use is disclosed. Further, a dosage form, including such form particularly adapted to producing an immune response, comprising dimyristoylphosphatidylcholine (DMPC) / cholesterol liposomes, optionally in an aluminum hydroxide gel, and an immunogen wherein said DMPC / cholesterol and immunogen are present in an immunization dose, and method according to use.
Owner:TRANSAVE
Treatment of gastro-intestinal disorders
InactiveUS6645530B1Dampen bacterial inactivationAcid secretion in the stomach could also be pharmacologically suppressedBiocideMilk preparationEscherichia coliDisease
A method of treating chronic disorders associated with the presence of abnormal microflora or an abnormal distribution of microflora in the gastrointestinal tract involves removing the host's existing enteric microflora and substitution of feces from a disease screener donor or composition comprising microorganism selected from the group consisting of Bacteroides and E. coli.
Owner:CRESTOVO LLC
Pharmaceutical tablet, completely coated, for controlled release of active principles that present problems of bio-availability linked to gastro-intestinal absorption
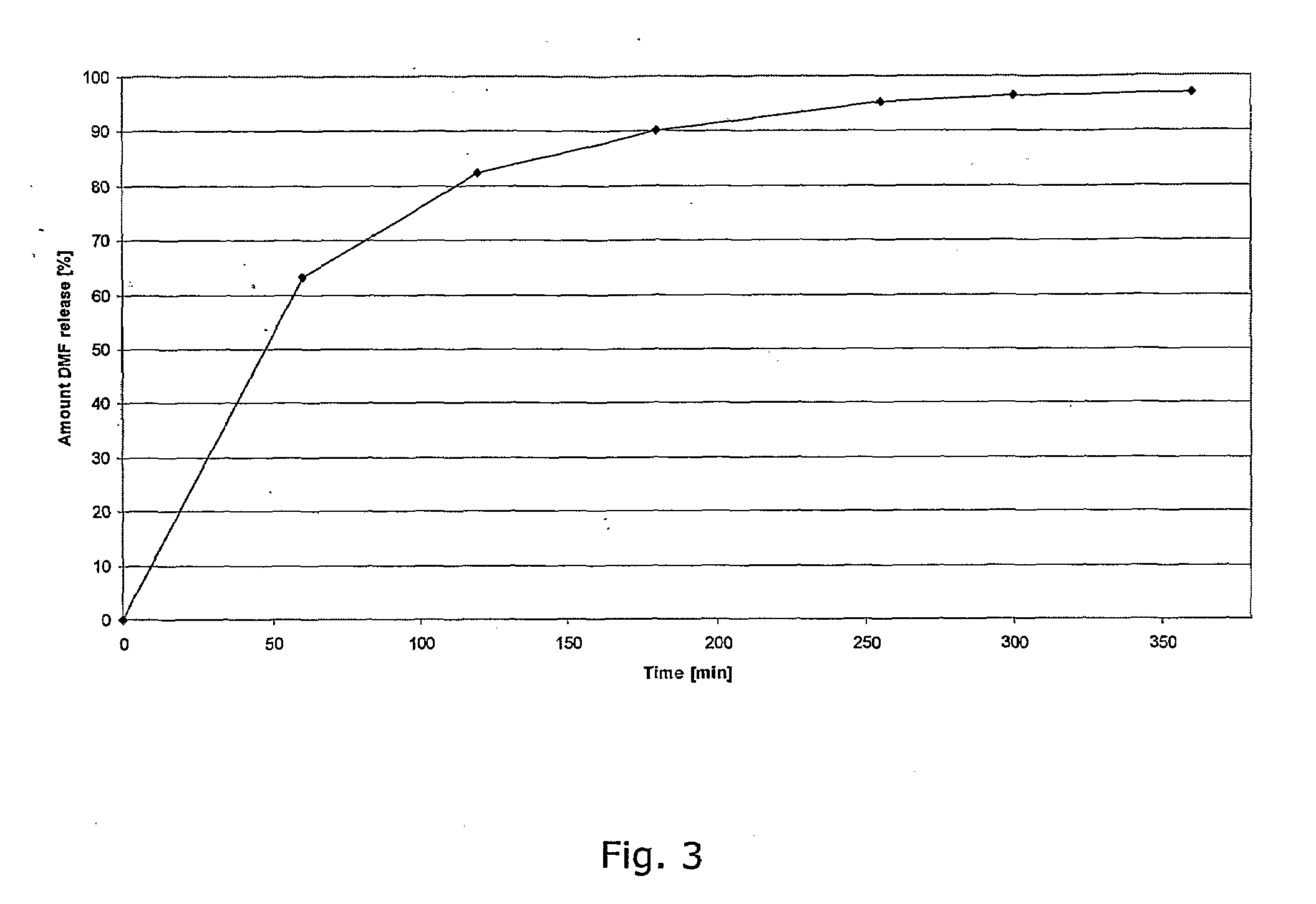
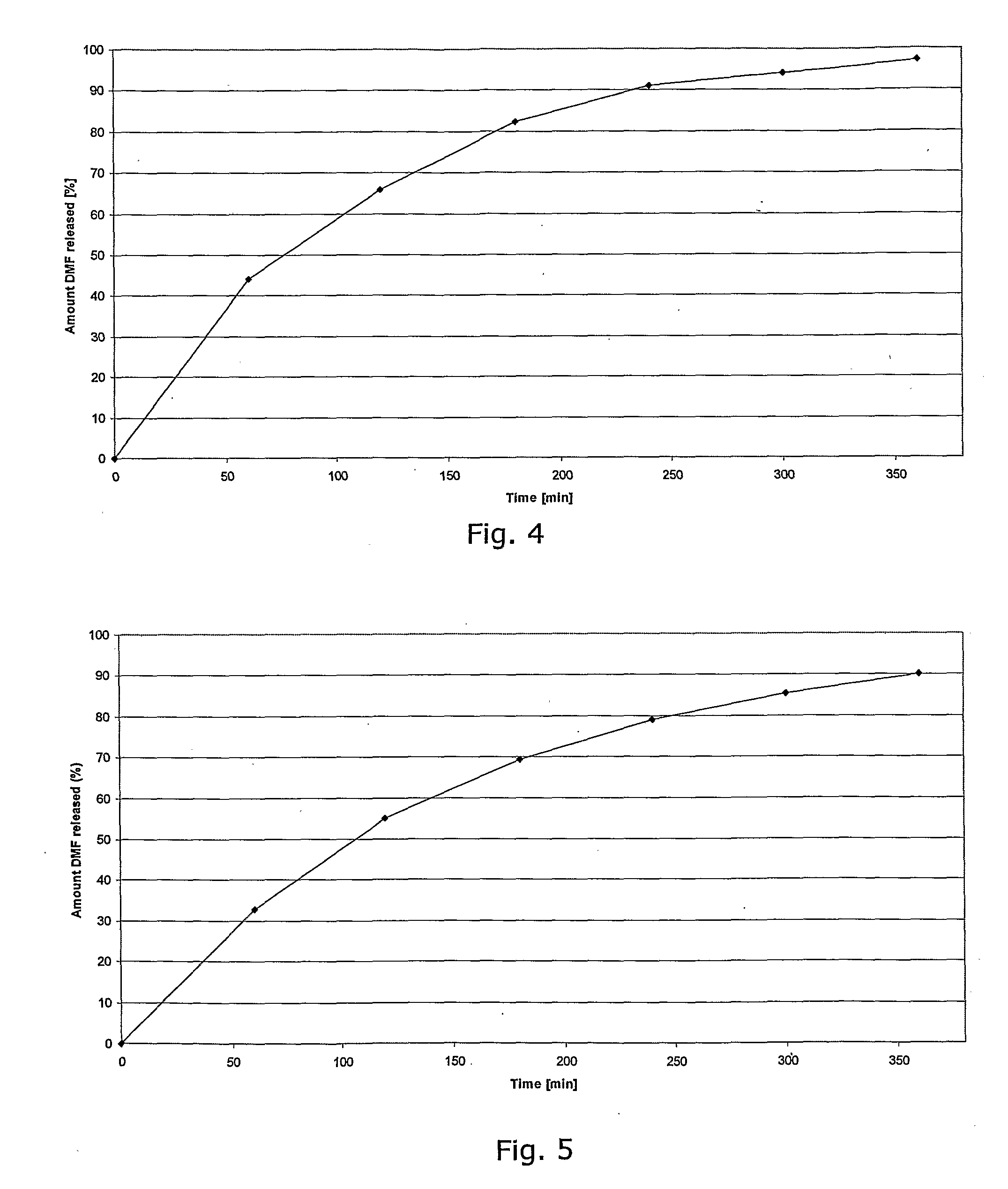
Described herein is a particular type of pharmaceutical tablet, for oral use, which is formed by one or more layers, and is specifically designed for controlled release of active principles that present problems of bio-availability linked to absorption in the gastro-intestinal tract, and in particular active principles that present an erratic and unpredictable absorption linked to the presence or absence of food at the level of the stomach and / or of the first portion of the small intestine, the said pharmaceutical form being characterized in that it is completely coated with one or more films of a biocompatible and biodegradable polymeric material.
Owner:JAGOTEC AG
Method of reducing ecologically adverse changes of the gastro intestinal microbial flora in patients under treatment with medicaments
InactiveUS20020022019A1Reduce generationBiocideOrganic active ingredientsMicroorganismPresent method
A method for reducing ecologically adverse changes of the gastrointestinal micro-flora in patients under treatment with medicaments (which may also be referred to herein as the therapeutic compounds or medications) such as gastric acid reducing medicaments or antibiotics. A pharmaceutical product useful in the present method comprising a medicament and a probiotically active organism as a combined preparation presented in a commercial package unit.
Owner:CHR HANSEN AS
Intracutaneous injection
ActiveUS20060211982A1Minimize injectionMinimize medicationPeptide/protein ingredientsAutomatic syringesSlurryIntracutaneous injection
The delivery of biopharmaceutical and other therapeutic agents parenterally to an animal via a minimally invasive, low pain administration is provided. The agents are delivered to the patient via, e.g., the epidermal, dermal, or subcutaneous layer of the skin in a concentrated form of injectable paste of slurry.
Owner:XERIS PHARMA
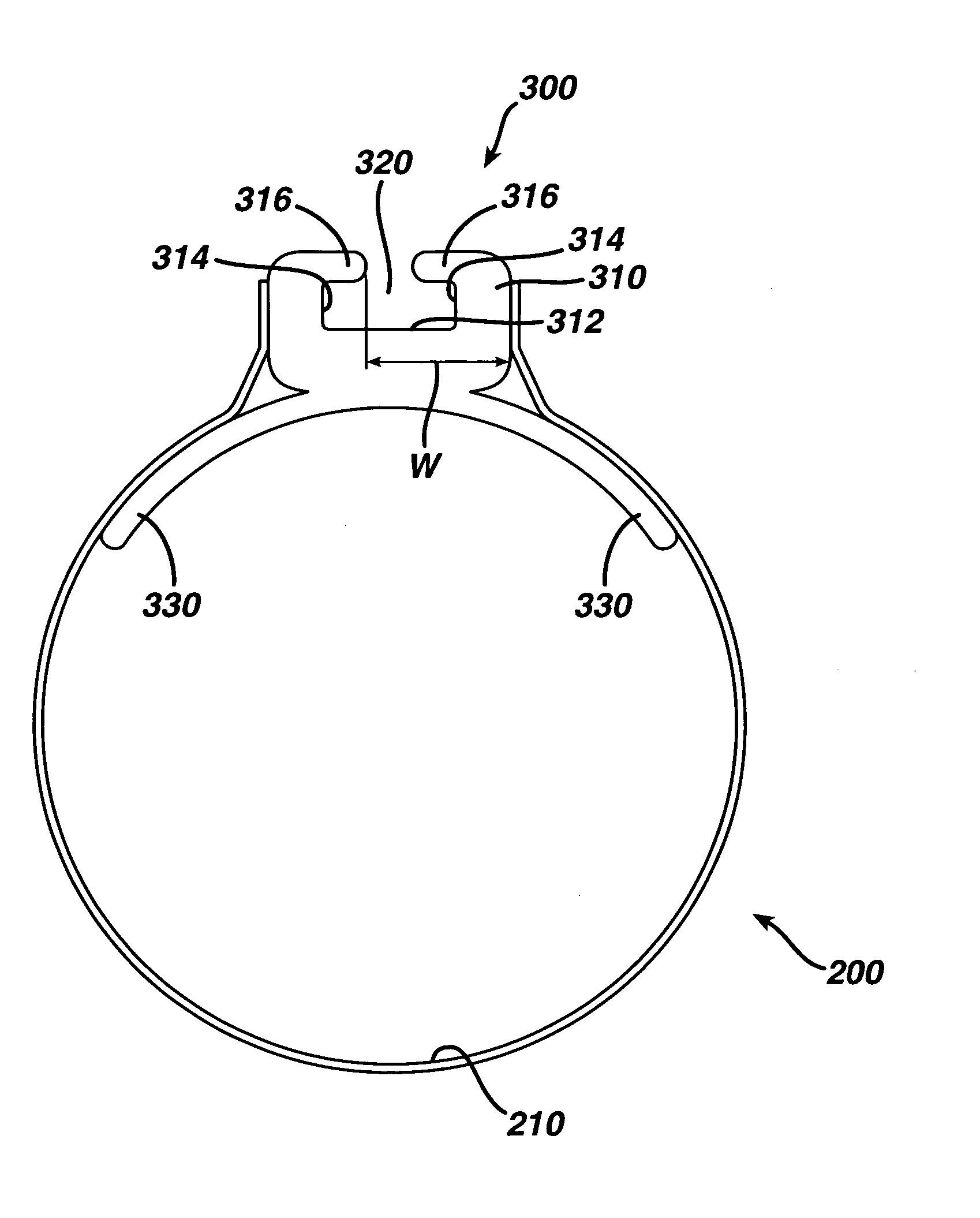
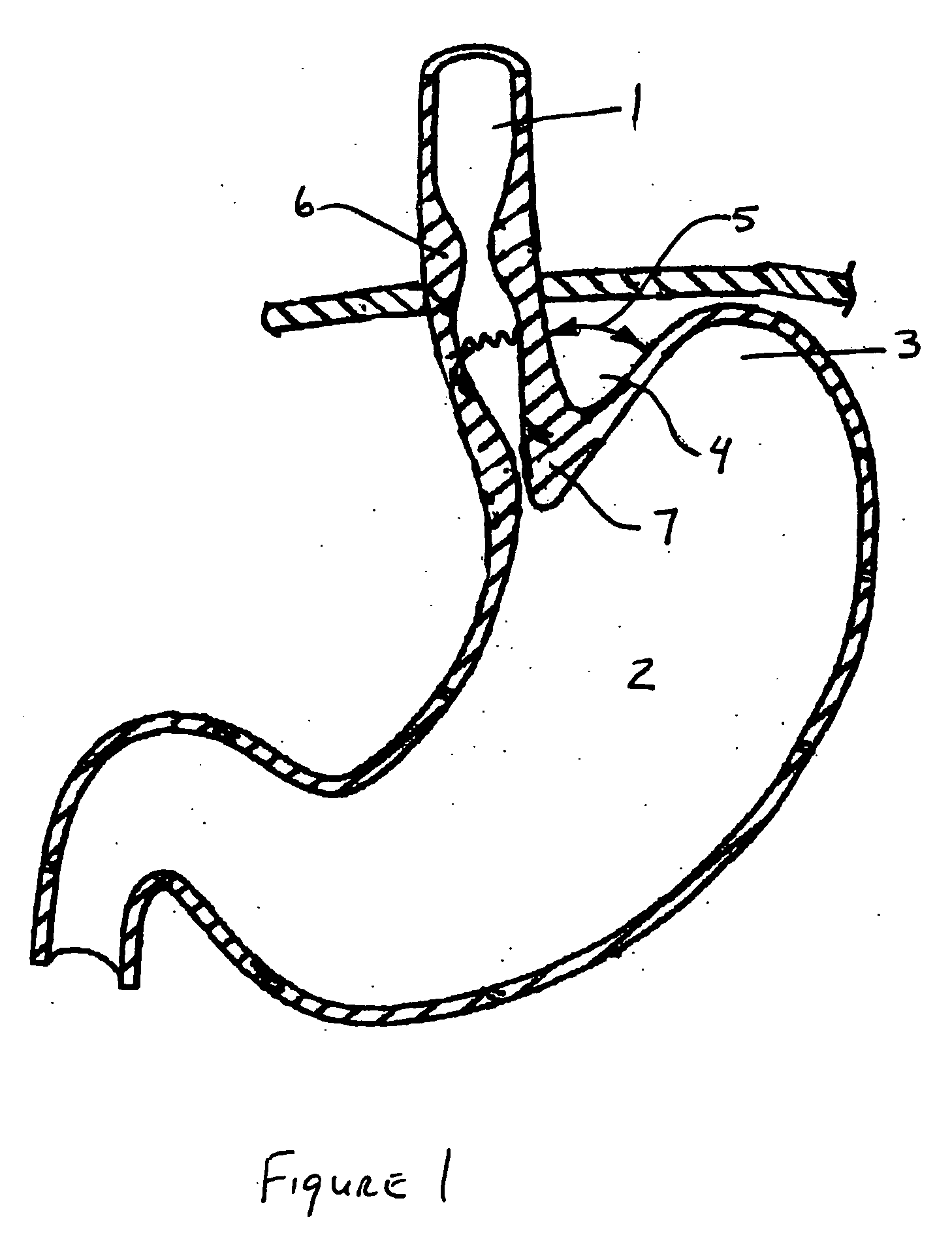
Non-invasive measurement of fluid pressure in an adjustable gastric band
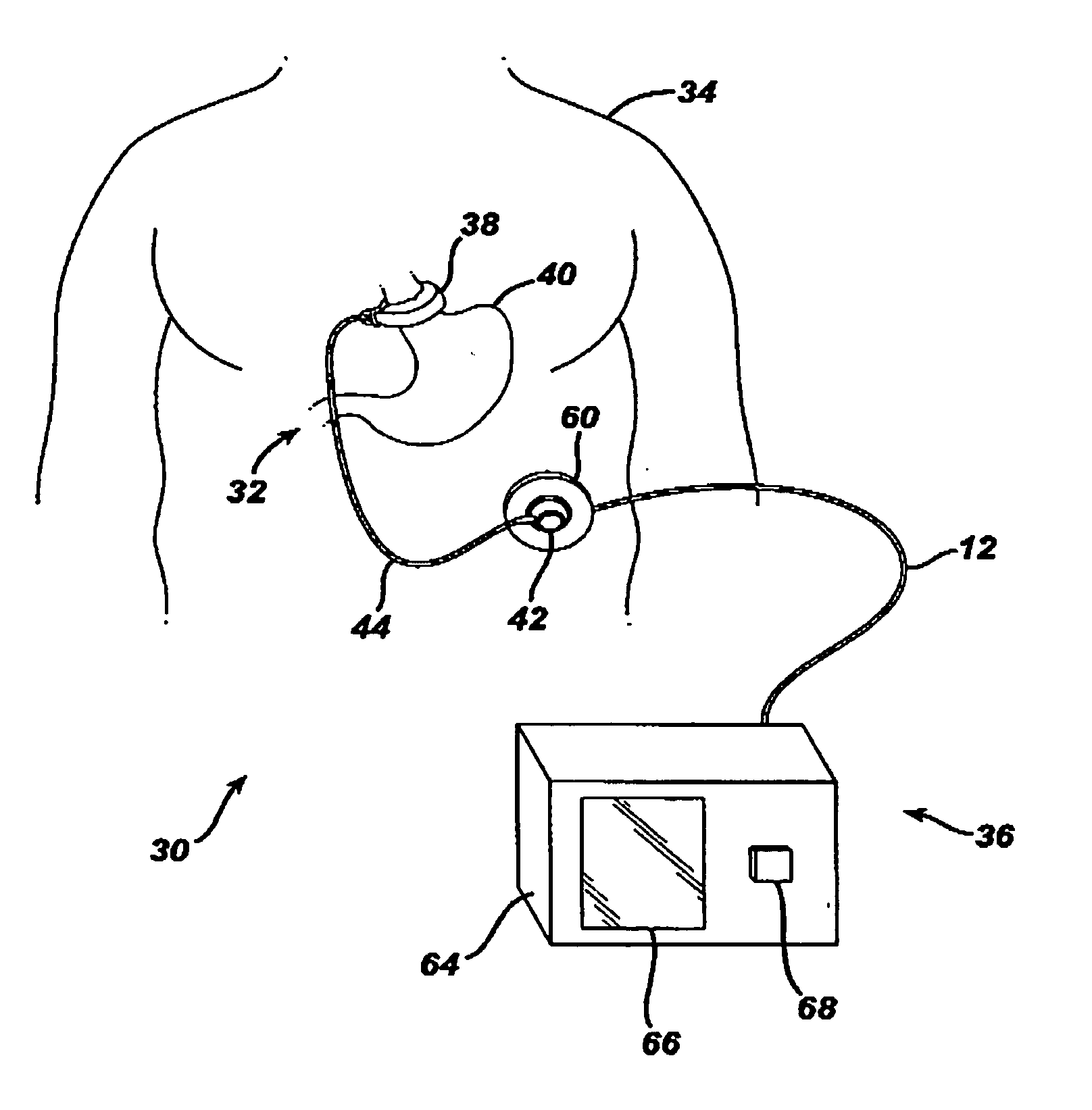
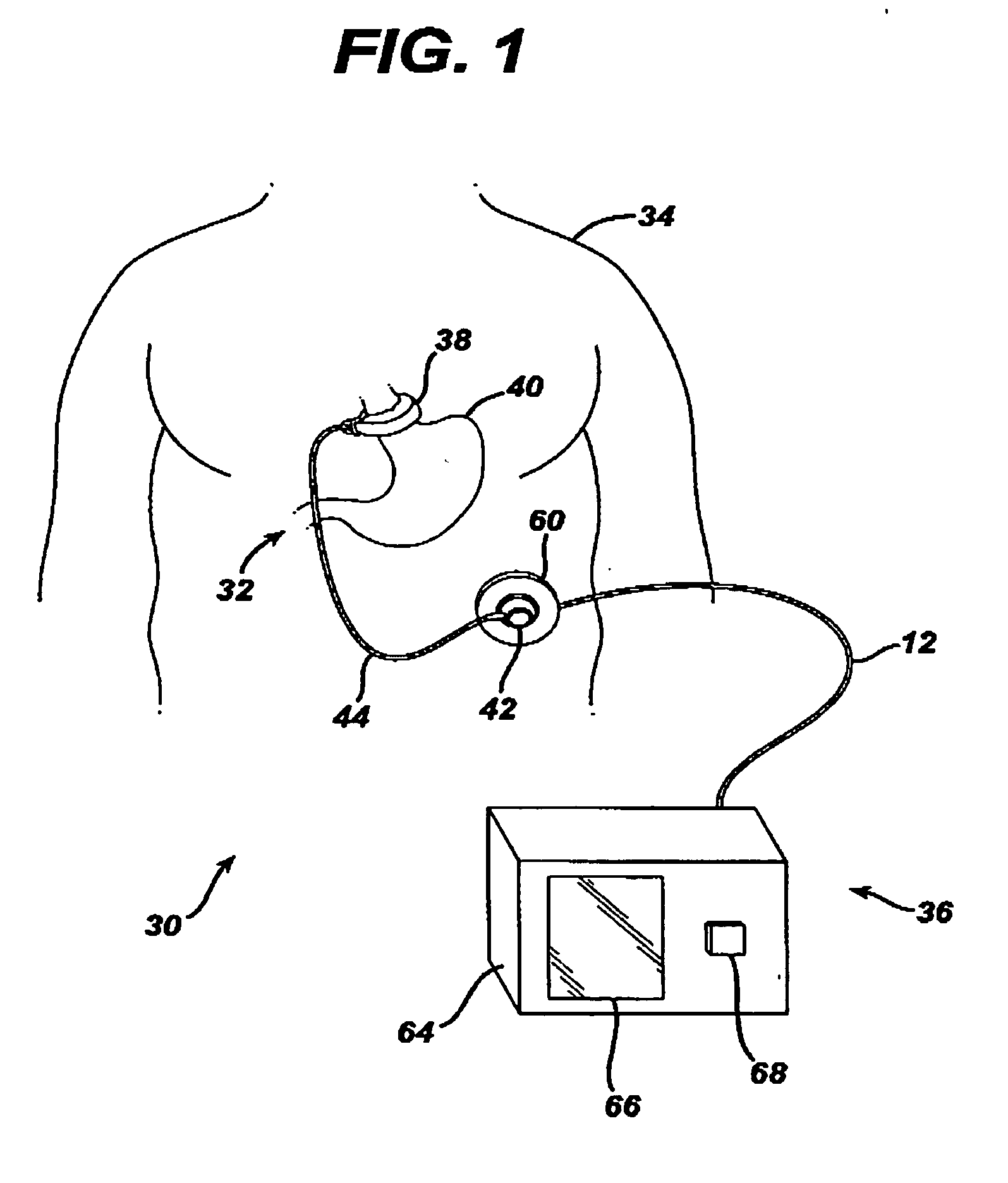
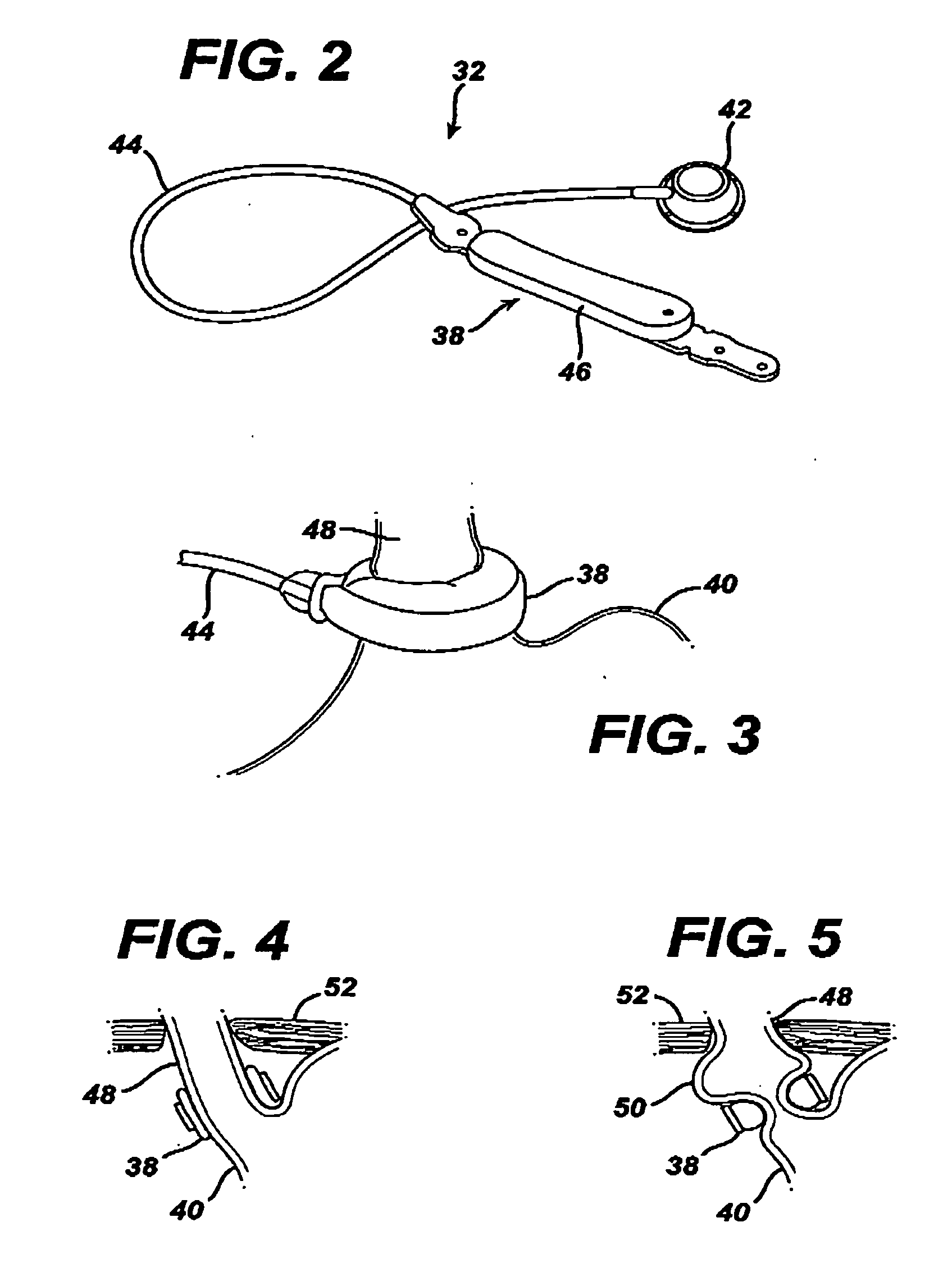
A food intake restriction device for forming a restriction in a patient's gastro-intestinal tract and non-invasively communicating pressure data regarding the restriction to an external monitor. The device includes a food intake restriction device implanted substantially about a patient's gastro-intestinal tract to form a restricted opening in the tract. A port is connected to the restriction device. The port contains a working fluid for affecting the size of the restricted opening. A pressure sensing system communicates with the working fluid to measure the pressure of the working fluid. A transmitter communicates the measured fluid pressure to the external monitor.
Owner:ETHICON ENDO SURGERY INC
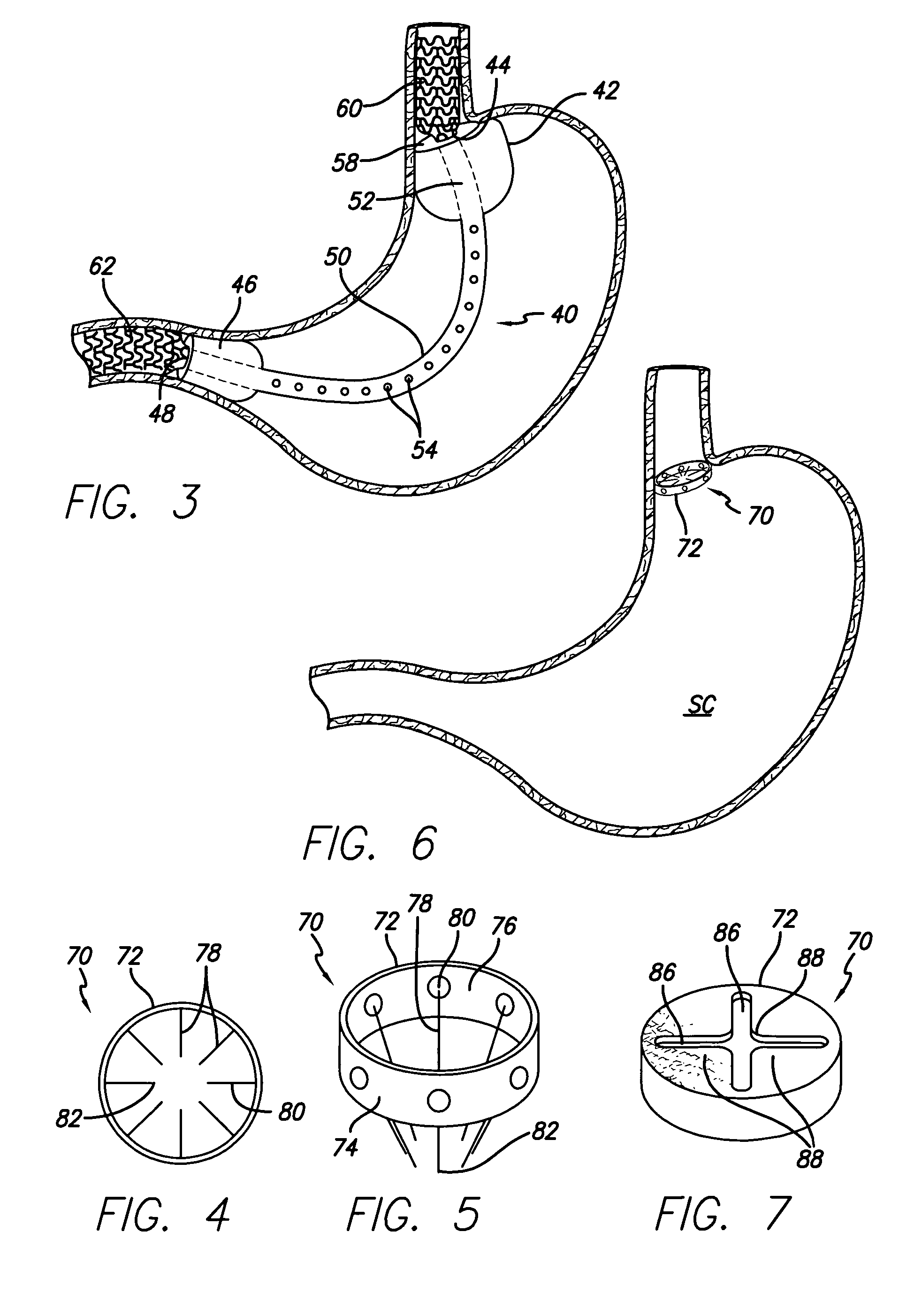
Systems and methods for treating obesity
ActiveUS7753870B2Reduce food intakeGood for weight lossIntravenous devicesTubular organ implantsPylorusGastric emptying
Methods and devices for simulating a gastric bypass and reducing the volume of the stomach involve placing a tubular liner along the lesser curve of the stomach cavity. Also, methods and devices for slowing gastric emptying involve placing valves within the stomach cavity near the gastro-intestinal junction and / or the pylorus. These methods and devices may prevent a patient from drinking and eating large volumes at one time and from eating slowly all day.
Owner:ETHICON ENDO SURGERY INC
Self-propelled, intraluminal device with working channel and method of use
InactiveUS6866626B2Minimize contractile forceEliminate needSurgeryEndoscopesSurgical deviceBody cavity
The present invention provides apparatus and method for providing access of a medical instrument, such as a surgical instrument, from a point outside the patient's body to a point within a body lumen, such as a portion of the Gastro-intestinal tract. A self propelled device, such as a capsule having electrodes for providing contraction of lumen tissue, can be positioned within the body lumen at a desired location. The medical instrument can be directed through a working channel associated with the capsule to access the body lumen tissue through the capsule.
Owner:ETHICON ENDO SURGERY INC
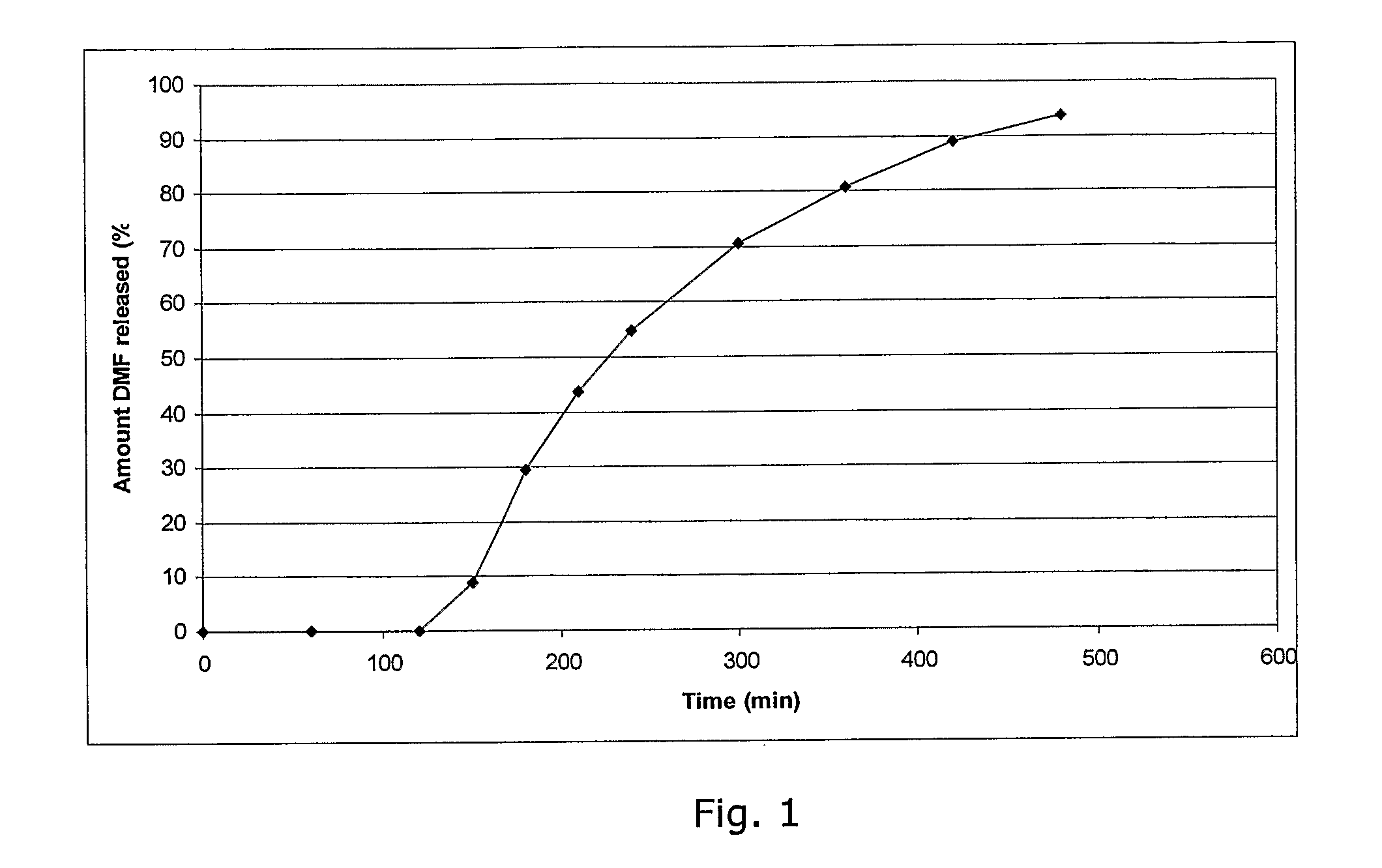
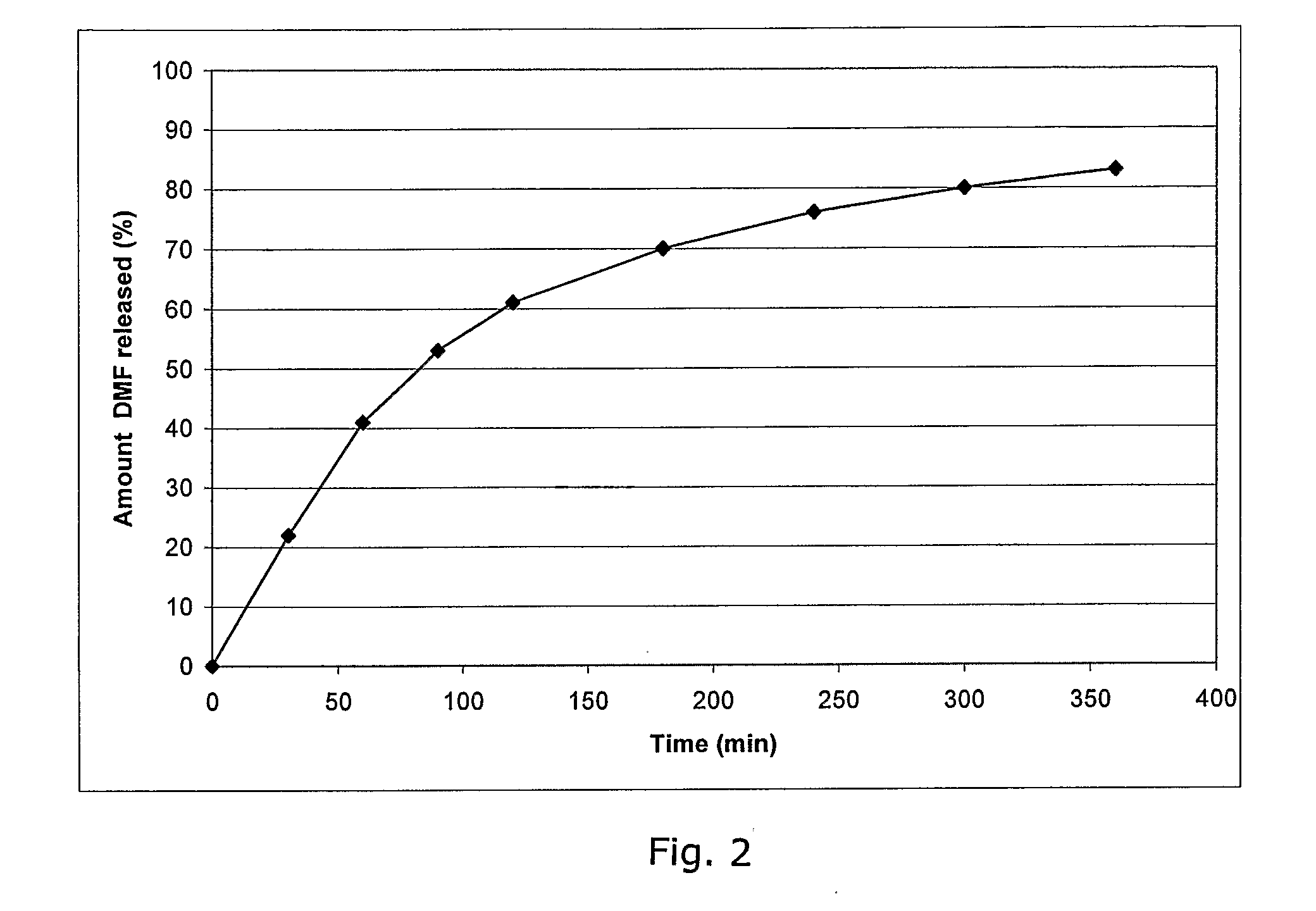
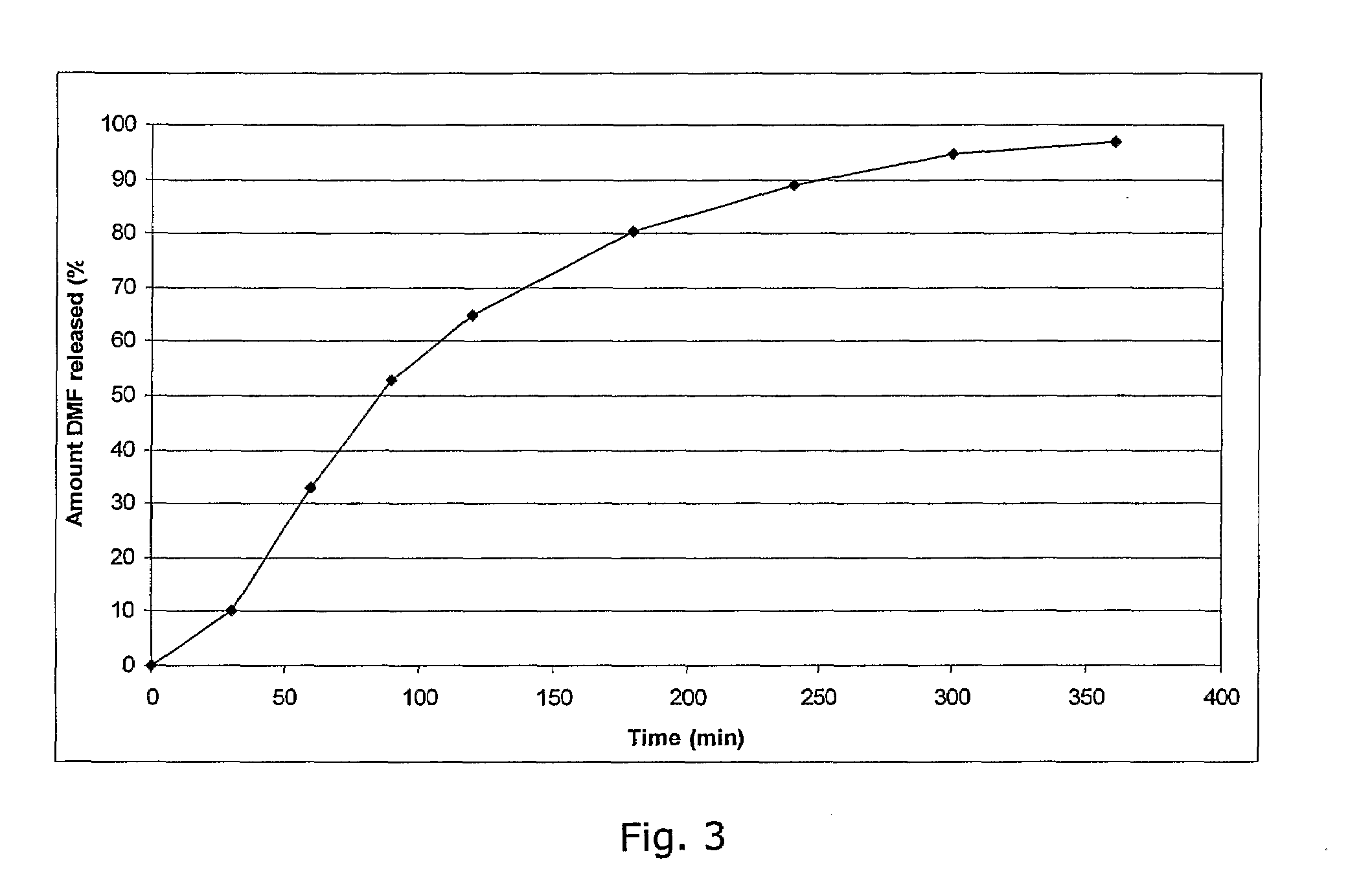
Controlled release pharmaceutical compositions comprising a fumaric acid ester
The present invention relates to controlled release pharmaceutical compositions comprising fumaric acid ester(s) as active substance(s). The compositions are suitable for use in the treatment of e.g. psoriasis or other hyperproliferative, inflammatory or autoimmune disorders and are designated to release the fumaric acid ester in a controlled manner so that local high concentrations of the active substance within the gastrointestinal tract upon oral administration can be avoided and, thereby, enabling a reduction in gastro-intestinal related side-effects.
Owner:BIOGEN SWISS MFG GMBH
Gastro-intestinal device and method for treating addiction
ActiveUS20070178160A1Prevents patient tamperingReduce appetitePowder deliveryElectrotherapyGastrointestinal deviceHazardous substance
A device and a method for treating a medical condition include a reversible member disposed in a patient's gastro-intestinal tract, and a dispensing member coupled to the reversible member that delivers a drug and / or a noxious when a predetermined substance is detected. In a different embodiment, the device and method of the present invention include a polymer infused with a drug and disposed into a preformed shell inside the gastric space, where it expands and hardens, releasing the drug over time. Both the casing and the polymer may be biocompatible. The present invention enables the slow-release of anti-addictive agents without patient tampering and with the appropriate dosage. Ancillary systems such as sensors, actuators, refill and recharge ports, and communication and data processing units may also be included.
Owner:BARONOVA
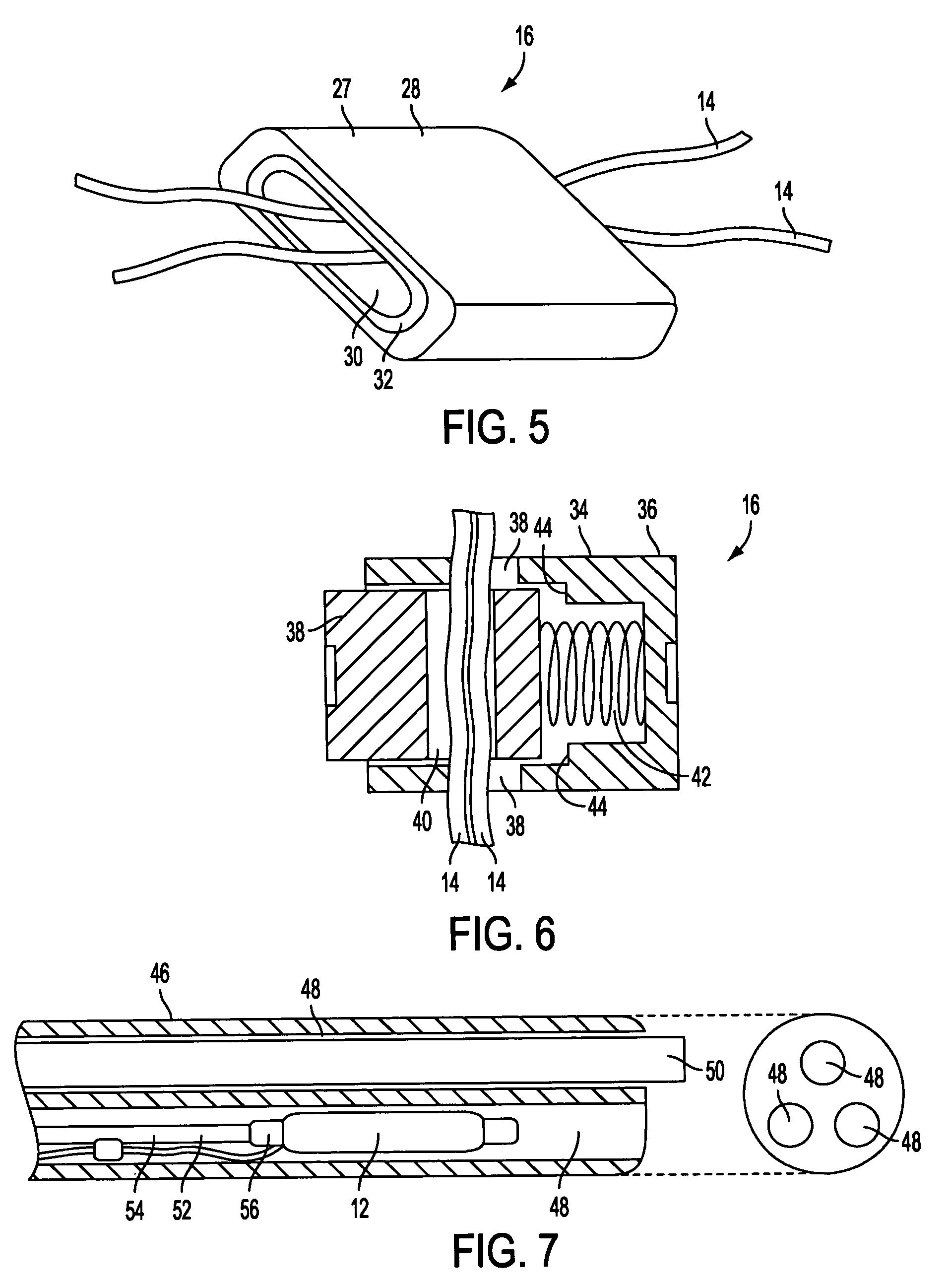
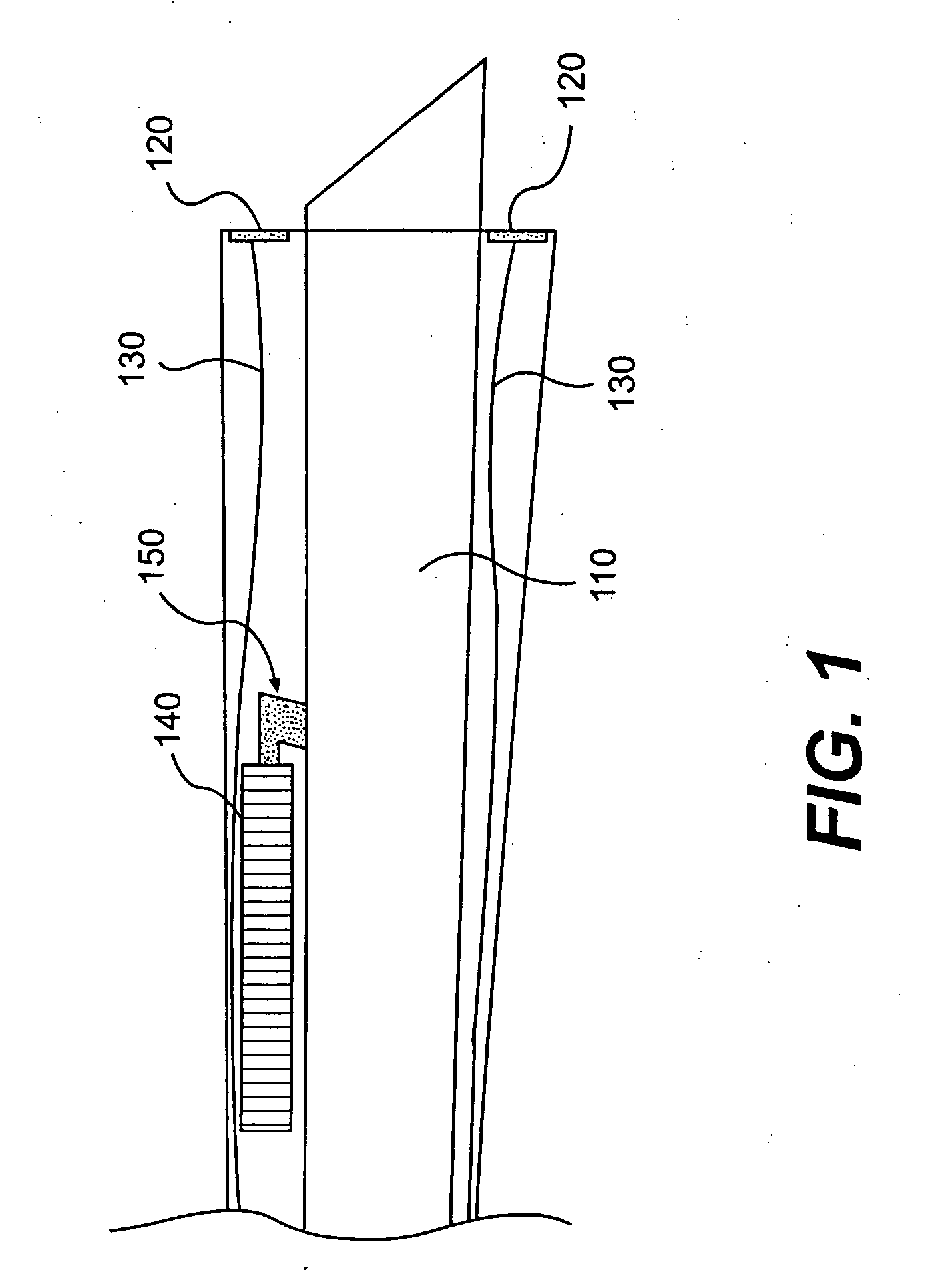
Sheath for use with an endoscope
ActiveUS20060258908A1Reduce in quantityQuickly and consistentlyGastroscopesCannulasEndoscopeMedical device
A medical apparatus and method useful for positioning one or more members within the gastro-intestinal tract is disclosed. The sheath can be provided with texture on an inside surface to facilitate installation of the endoscope in the sheath, and to permit the endoscope to be gripped through the sheath.
Owner:ETHICON ENDO SURGERY INC
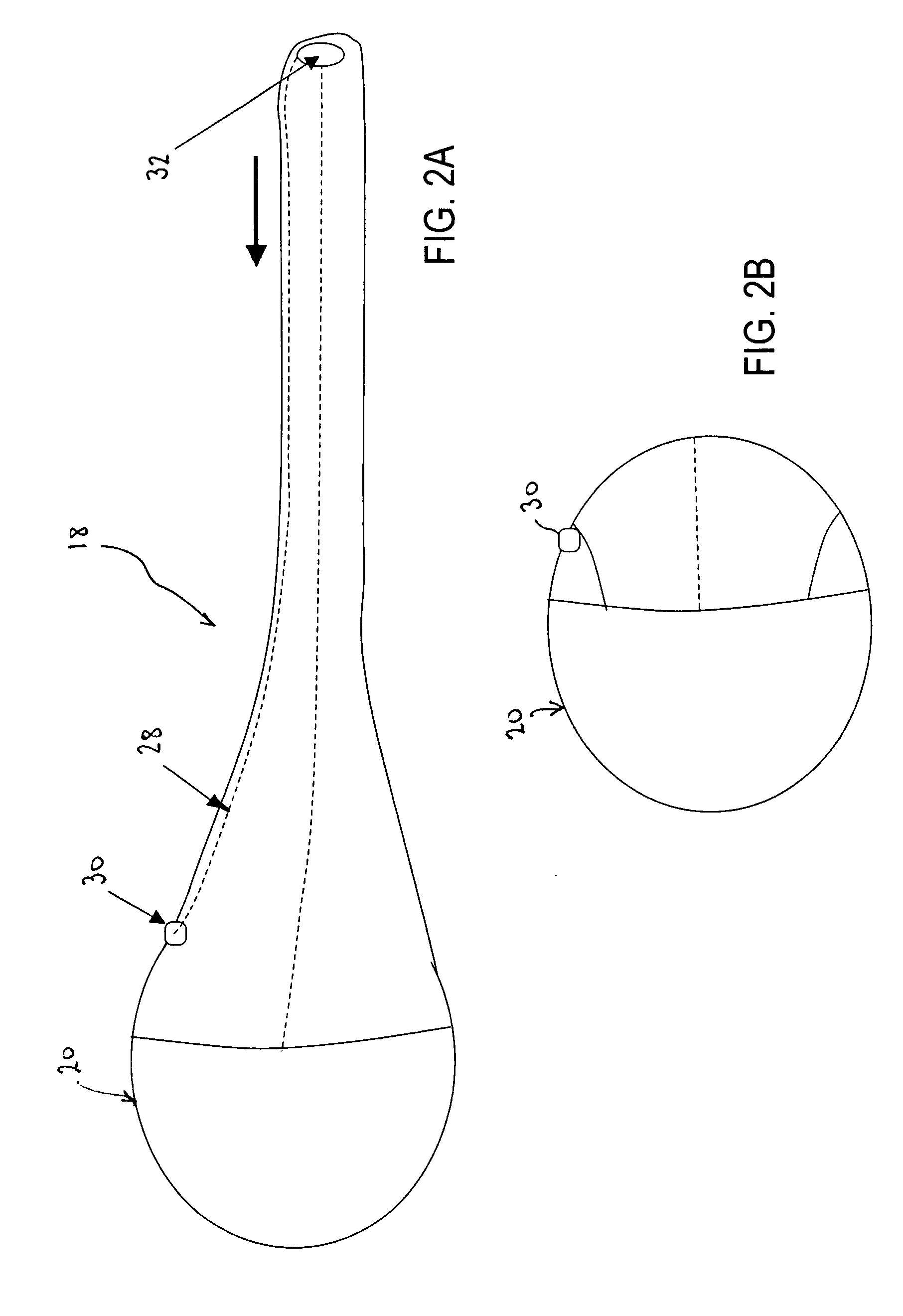
Track for medical devices
ActiveUS20060258907A1Reduce in quantityQuickly and consistentlyGastroscopesCannulasMedical deviceFeeding tube
A medical apparatus and method useful for positioning one or more members within the gastro-intestinal tract is disclosed. The medical apparatus can include a track supported on a sheath sized to receive an endoscope, and a carrier slidable with respect to the track. A feeding tube accessory adapted to slidably engage the carrier is disclosed.
Owner:ETHICON ENDO SURGERY INC
Treatment of inflammatory conditions of the intestine
Owner:CSL BEHRING AG
System and method for mapping gastro-intestinal electrical activity
InactiveUS20130035576A12D-image generationSurgical instrument detailsSmooth muscleBiological activation
A gastro-electrical activity mapping system and comprises a catheter insertable through a natural orifice into the gastro-intestinal (GI) tract and comprising an array of electrodes for contacting an interior surface of a section of the GI tract to detect electrical potentials at multiple electrodes, and a signal analysis and mapping system arranged to receive and process electrical signals from multiple electrodes of the array and spatially map GI smooth muscle electrical activity as an activation time map, a velocity map, or an amplitude map, which may be in the form of contour plots and may be mapped on an anatomical computer model of at least the section of the GI tract and may be animated. A GI mapping method and catheter are also claimed.
Owner:AUCKLAND UNISERVICES LTD
Controlled Release Pharmaceutical Compositions Comprising a Fumaric Acid Ester
InactiveUS20080299196A1Decrease glass transition pointLow film forming temperatureBiocideSenses disorderDiseaseHigh concentration
The present invention relates to controlled release pharmaceutical compositions comprising fumaric acid ester(s) as active substance(s). The compositions are suitable for use in the treatment of e.g. psoriasis or other hyperproliferative, inflammatory or autoimmune disorders and are designed to release the fumaric acid ester in a controlled manner so that local high concentrations of the active substance within the gastrointestinal tract upon oral administration can be avoided and, thereby, enabling a reduction in gastro-intestinal related side-effects.
Owner:BIOGEN SWISS MFG GMBH
Compound vegetable healthcare product and method for preparing same
InactiveCN102228242ATo promote metabolismPromote peristalsisFood preparationBiotechnologyFood additive
The invention relates to a nutrient healthcare product and a method for preparing the same, in particular to a compound vegetable healthcare product and a method for preparing the same. The compound vegetable nutrient powder of the invention consists of the following components in part by weight: 0.1 to 100 parts of dried vegetable powder, 0 to 10 parts of alga-type plant regulating powder, 0 to 96 parts of auxiliary component, 0 to 5 parts of natural flavoring agent and 0 to 5 parts of food additive. The preparation method adopts a simple production process and is suitable for industrialized production, and the product prepared by the method is rich in nutrition and stable in quality, meets the demands of people on various nutrients, regulates the intestines and stomach, improves enterogastric peristalsis, enhances in vivo metabolism of sulfur-contained substances, avoids the adverse effects of human body caused by malnutrition and unbalanced nutrition and prevents and helps to treat diseases such as ulcerative stomatitis, diabetes and constipation. The product has also the advantages of convenience for carrying and long storage period.
Owner:肖天存
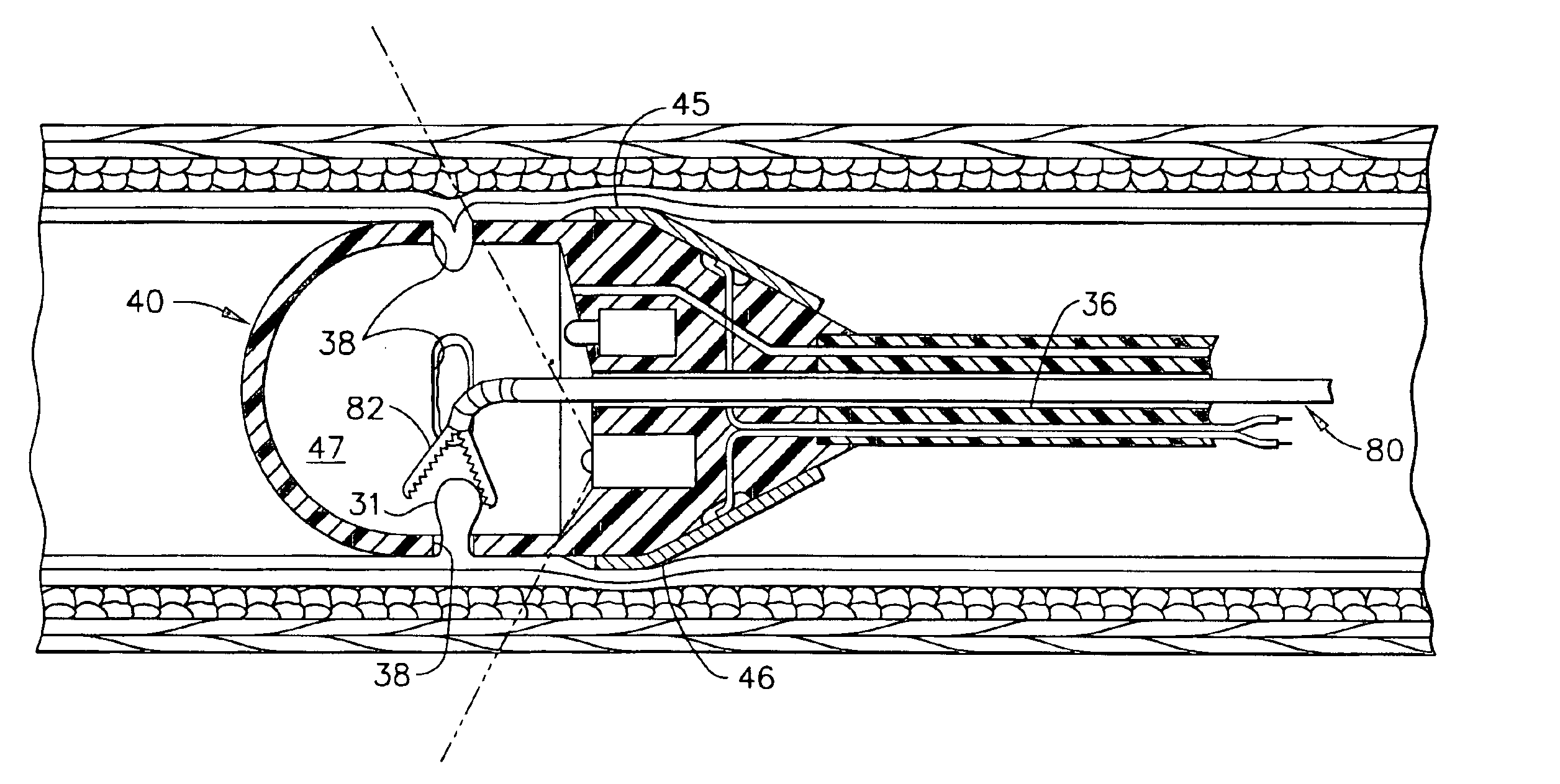
Methods and devices for anchoring to tissue
ActiveUS20070135825A1Reduce gastroesophageal refluxEasy to installSuture equipmentsSurgical needlesFlexible endoscopeCoupling
The present invention relates to a tissue securement system, device and method for endoscopy or endosonography-guided transluminal interventions whereby a ligation is placed and secured into tissue. An objective of this invention is to provide a stable securement platform for the coupling of secondary anchors to the gastro-intestinal tract. Specifically, endosonography is used to insert an anchoring element through the walls of adjacent body cavities. The ends of the anchoring element are coupled together to form a loop. This anchoring element can be used to secure other anchors or devices to the gastro intestinal tract.
Owner:ADVENT MEDICAL
Devices and methods for forming an anastomosis
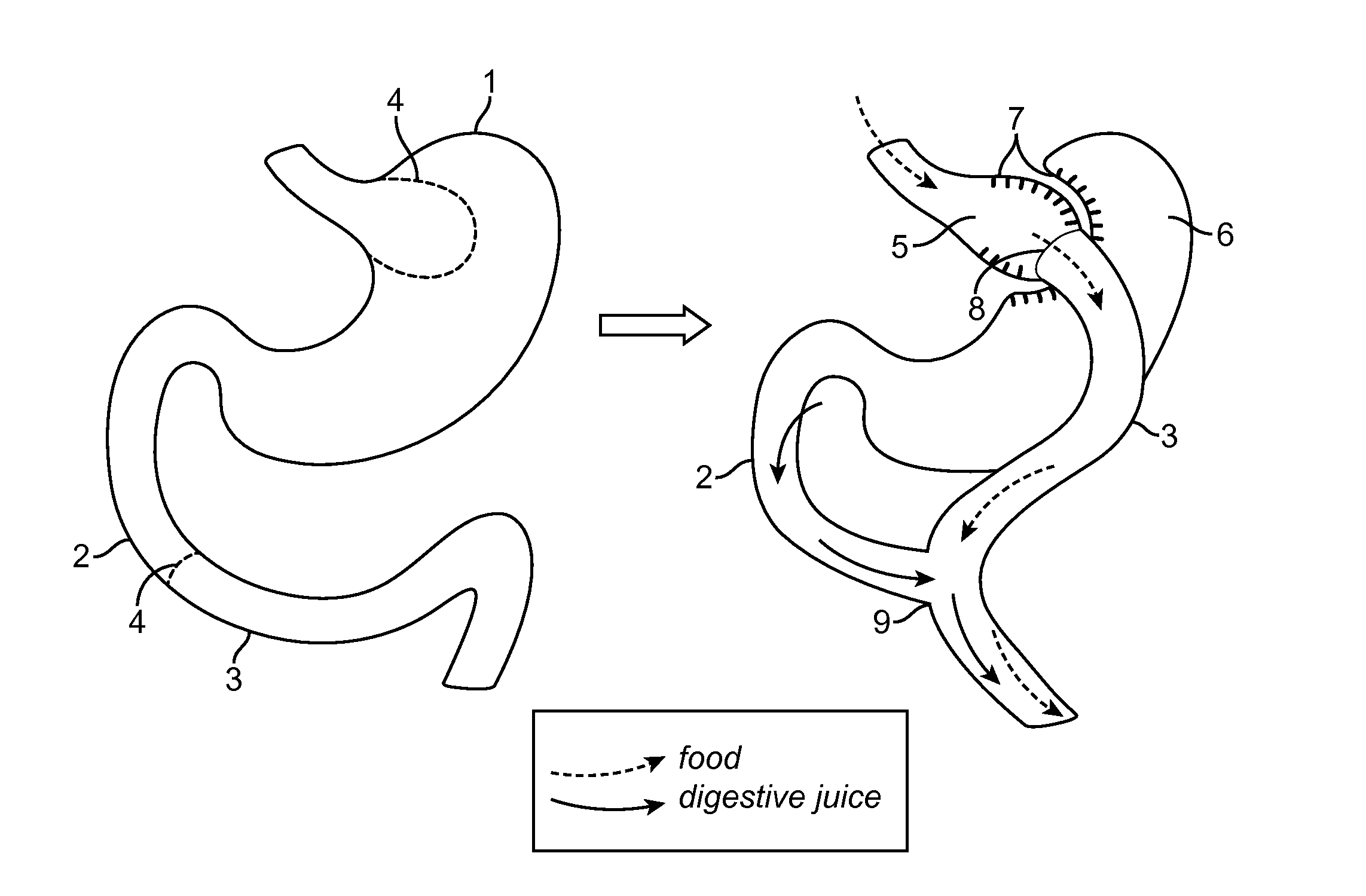
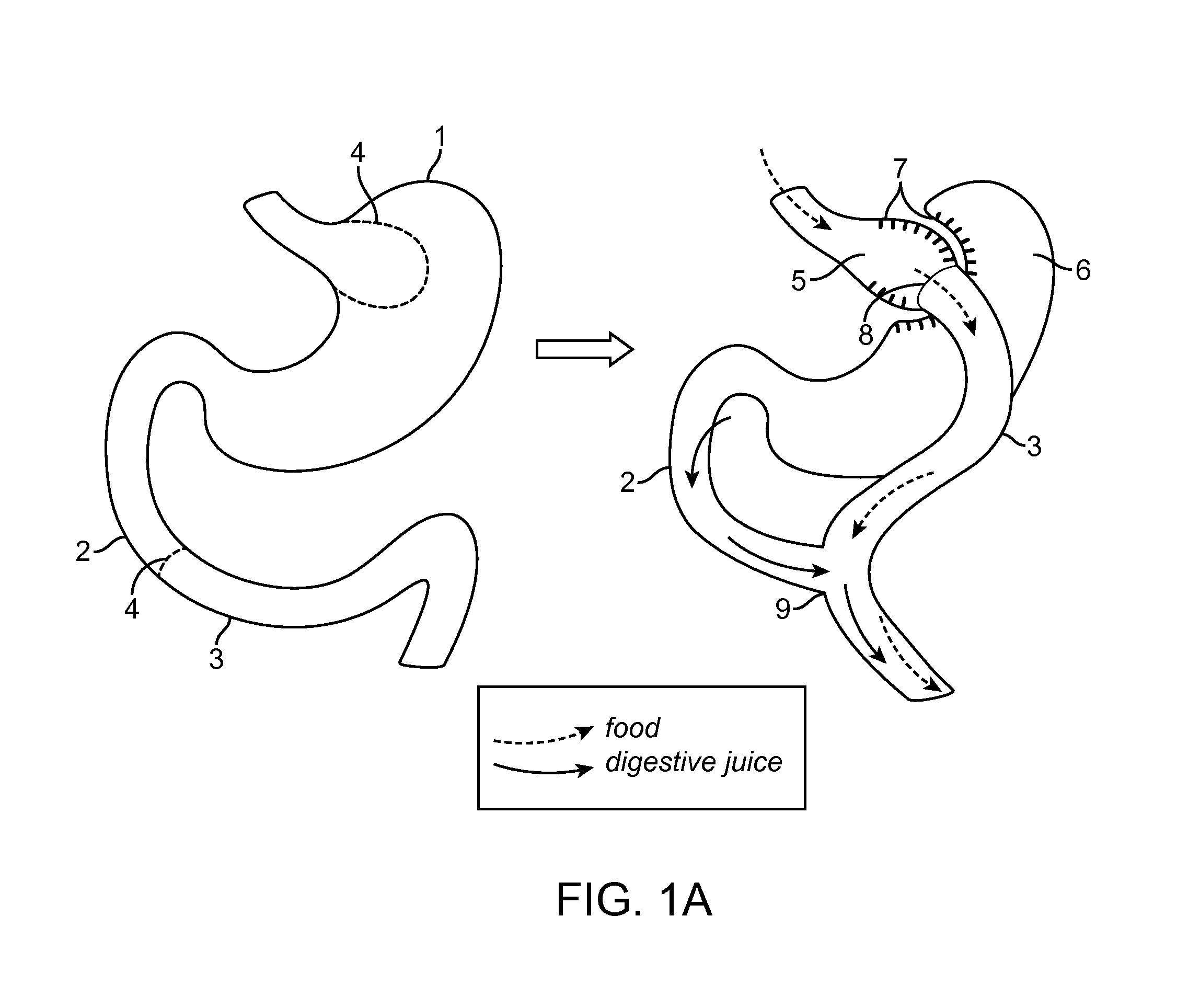
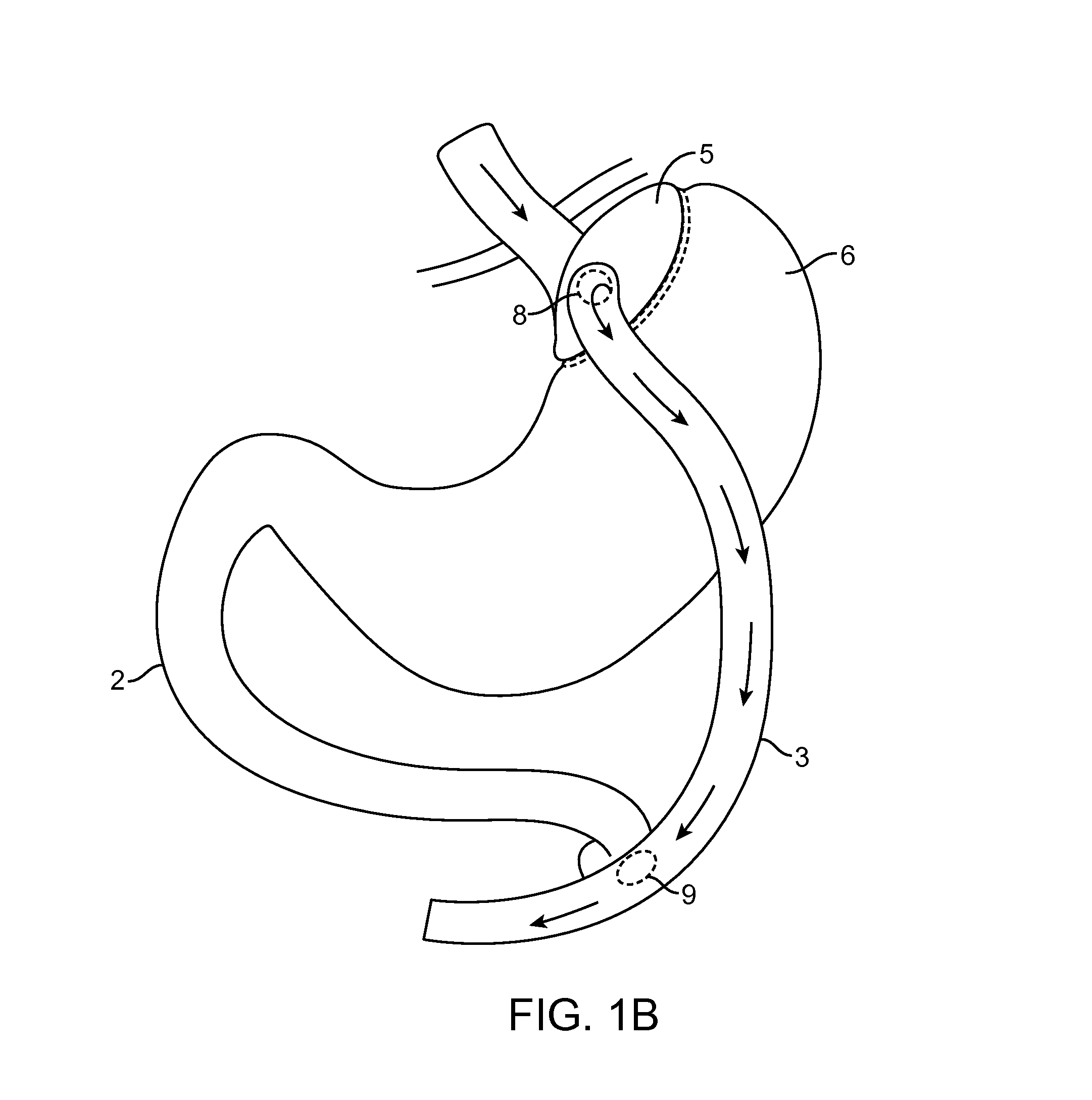
Devices and methods for deploying an anastomotic stent between portions of the gastro-intestinal (GI) tract are disclosed. The anastomotic stents are configured to atraumatically engage the tissue walls and to permit the flow of fluid, partially digested food, and food. The stents can be deployed using endoscopic catheter devices, laparoscopic tools, and combinations of both endoscopic tools and laparoscopic tools. Examples of anastomoses include anastomoses between the stomach and a portion of the intestines such as the jejunum. Anastomoses can also be formed between two closed ends of the intestines, such as two closed ends of the colon formed during a colon resection procedure. Anastomoses can also be formed between a fundal pouch formed during a gastric bypass procedure and the jejunum. Laparoscopic tools are disclosed to deploy a stent by selectively removing a radial restraint on a self expanding stent with the restraint removed through the laparoscopic access points.
Owner:BOSTON SCI SCIMED INC
Devices and methods for endolumenal therapy
InactiveUS20090093767A1Promoting tissue in-growthAltering abilityDiagnosticsSurgeryGastrointestinal deviceLower esophagus
The present invention is directed generically to a means for altering the ability of the mammalian body to absorb nutritive content from ingested foodstuffs, and more specifically to an apparatus and method of use for an endolumenal sleeve (referred to also as an “intragastrointestinal device” or “gastrointestinal device”) positioned in the mammalian gastrointestinal (GI) tract. A suitable endolumenal sleeve is comprised of an anchor element and an opening at a proximal end, an elongate lumen or hollow open-ended tube having a transverse dimension, and a distal orifice. Optionally, an exterior aspect of the elongate lumen may include additional modes of attachment to the tissues walls of the GI tract through the use of one or more means for promoting tissue in-growth. The endolumenal sleeve is retained in the GI tract such that a substantial fraction of the food and liquids passing through the GI tract is channeled into the proximal opening and through an interlumenal space defined within the interior space of the endolumenal sleeve. Within the endolumenal sleeve there may be one or more restrictive means to constrain, impede or otherwise control the operative flow of material through the device. An individual restrictive means can either be of a fixed geometry or such means may include one or more elements which are adjustable in nature or function. The elongate lumen of the endolumenal sleeve is formed of a polymer composition suitable for controlled ingress of biological secretions, egress of certain selected nutritional elements, and may comprise either a single tubular structure or a multi-section (i.e. articulated and / or multiple lumen) assembly. When the endolumenal sleeve is in situ within the mammalian gastro-intestinal system, ingested foodstuffs are conveyed from the proximal end to said distal orifice. In typical applications, the proximal end of the endolumenal sleeve is positioned within the physiological region extending from the lower esophagus to the duodenum and the distal orifice is positioned within the physiological region extending from the upper duodenum to the lower jejunum, though further extension into the lower intestine is possible. Through proper selection of position for the endolumenal sleeve proximal and distal ends, combined by selection of the composition used in the fabrication of the elongate lumen, it is possible to finitely control the degree of nutritive absorption performed by the gastrointestinal tract.
Owner:KELLEHER BRIAN
Method for treating oncological diseases
ActiveUS7612032B2Promote growthImprove efficiencyPeptide/protein ingredientsHydrolasesDiseaseWhole body
A method to treat solid tumors and other oncological diseases consists of parenterally injecting an agent which destroy's blood's extracellular DNA into the systemic blood circulation of a cancer patient to slow down malignant. The agent is embodied in the form of a DNAse enzyme and, more particularly, as a bovine pancreatic DNAse. Doses from 50,000-250,000,000 Kunz units / day are injected for 5-360 days. A binding agent or an agent that modifies the chemical composition of the blood extracellular DNA is additionally injected into the blood. This modifying agent is preferably an enzyme-ribonuclease.
Owner:CLS THERAPEUTICS
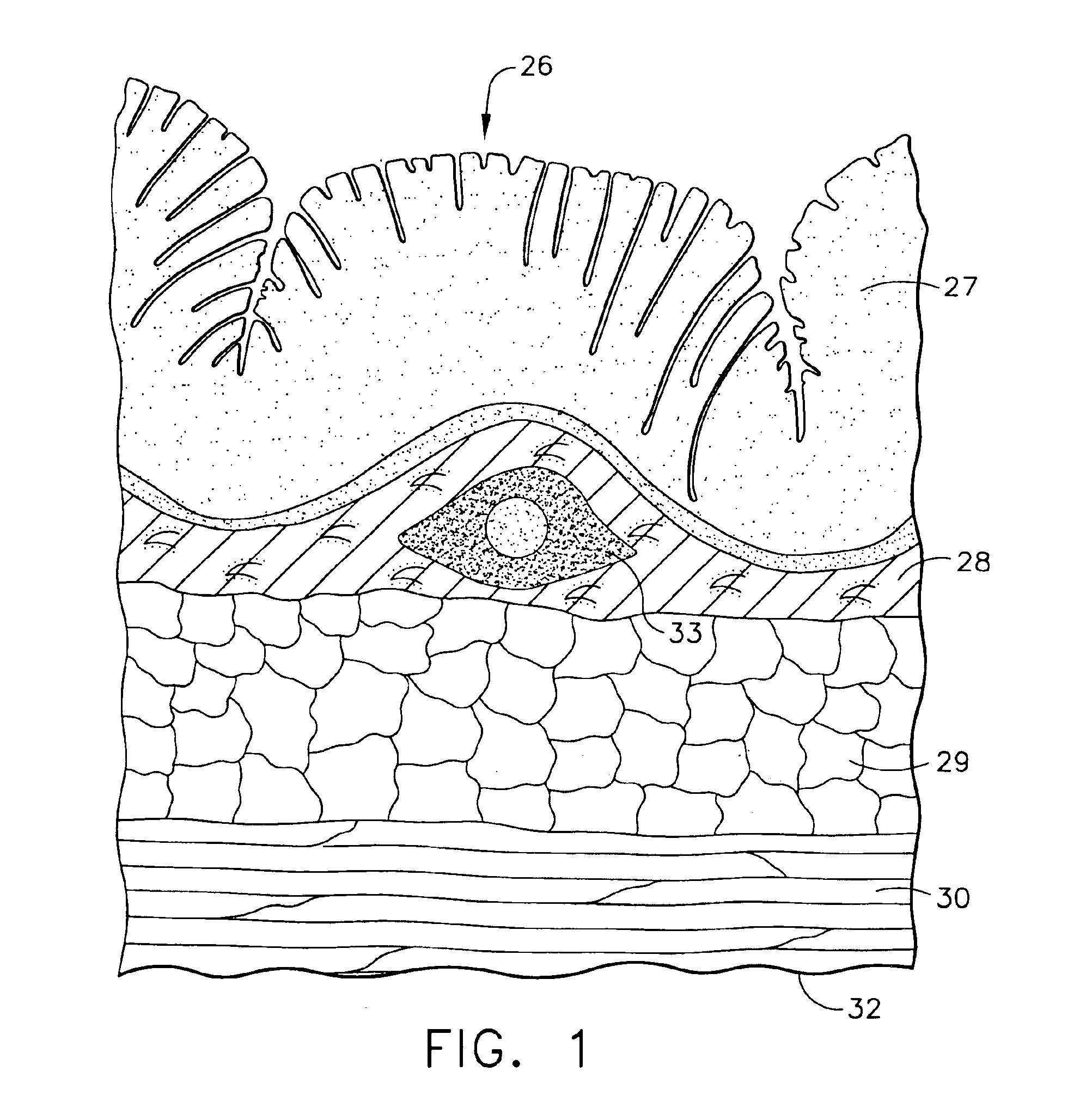
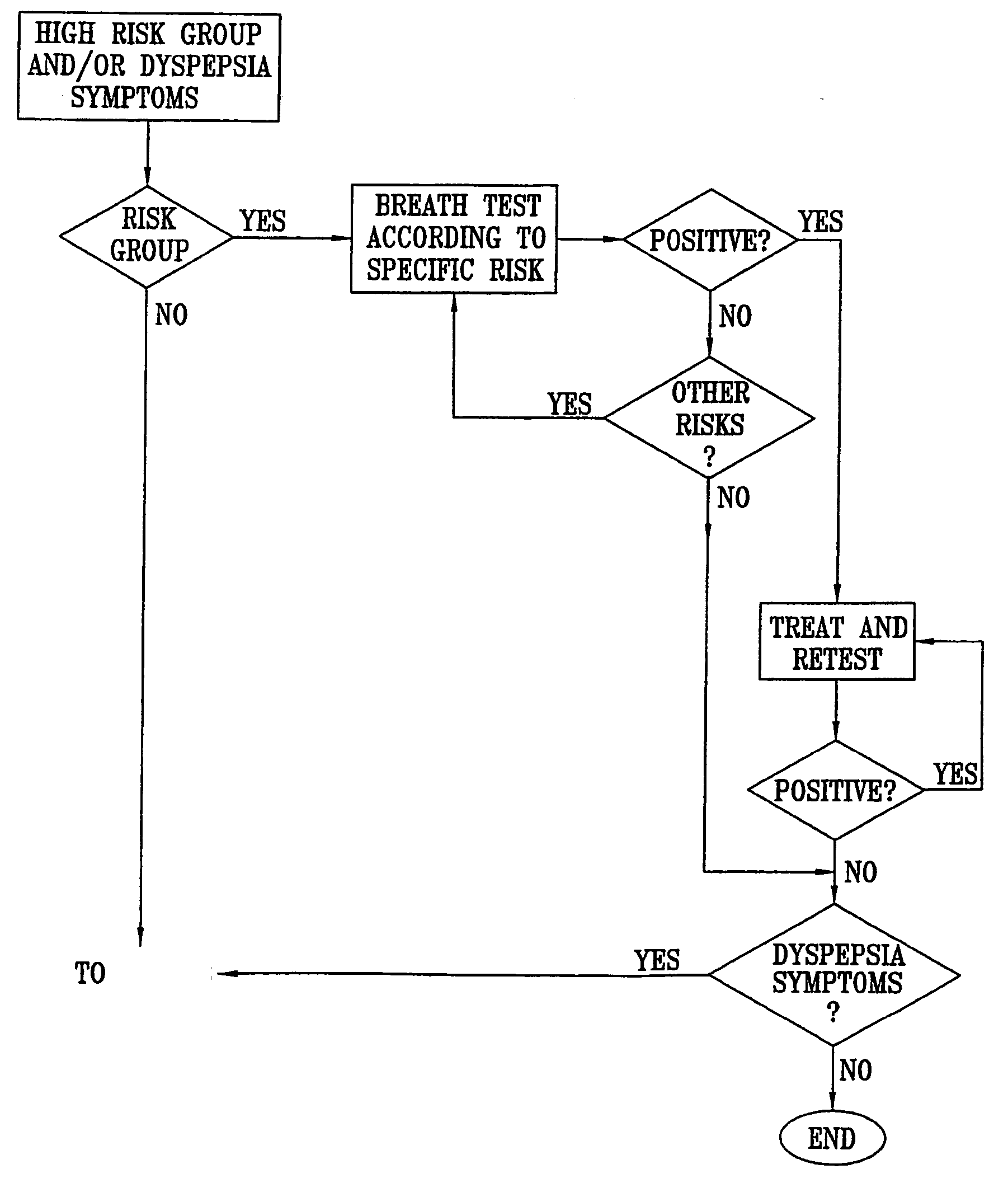
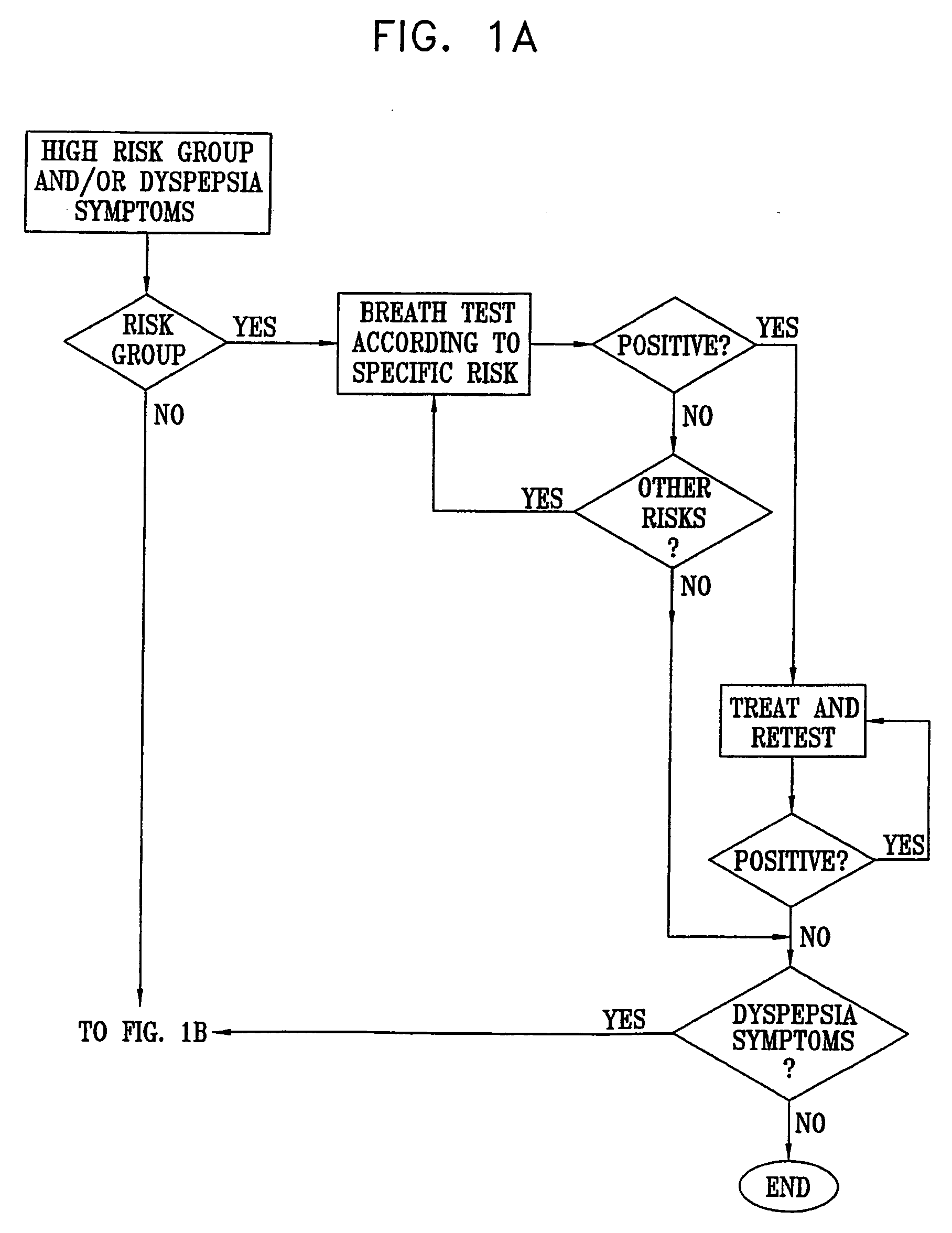
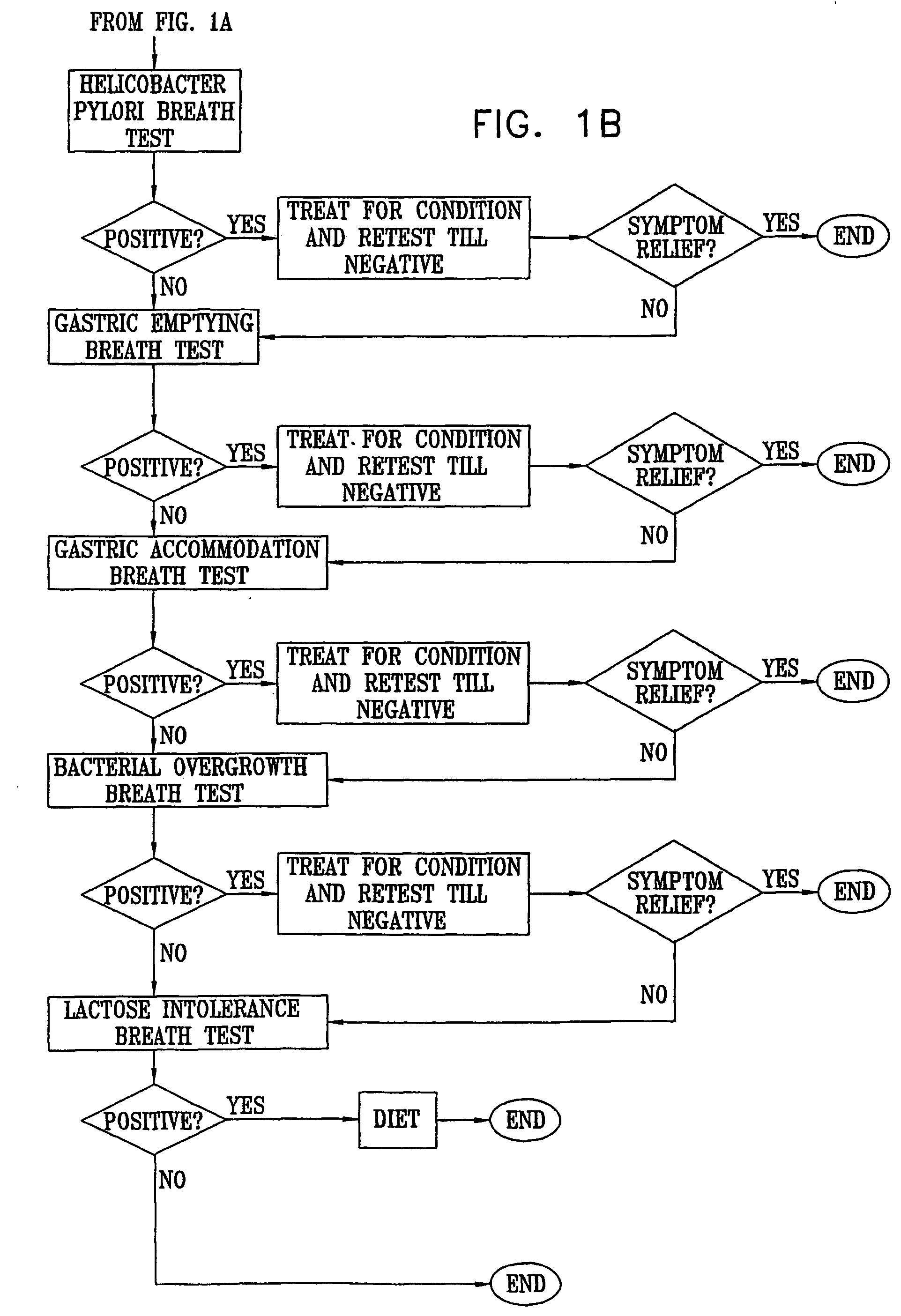
Management of gastro-intestinal disorders
InactiveUS20060074335A1Easily toleratedQuick testCompounds screening/testingPerson identificationDiseaseGastrointestinal dysfunction
The present invention relates to the field of methods and apparatus for the determination of various conditions of gastric and gastro-intestinal malfunction, especially those performed by means of breath tests.
Owner:EXALENZ BIOSCIENCE LTD
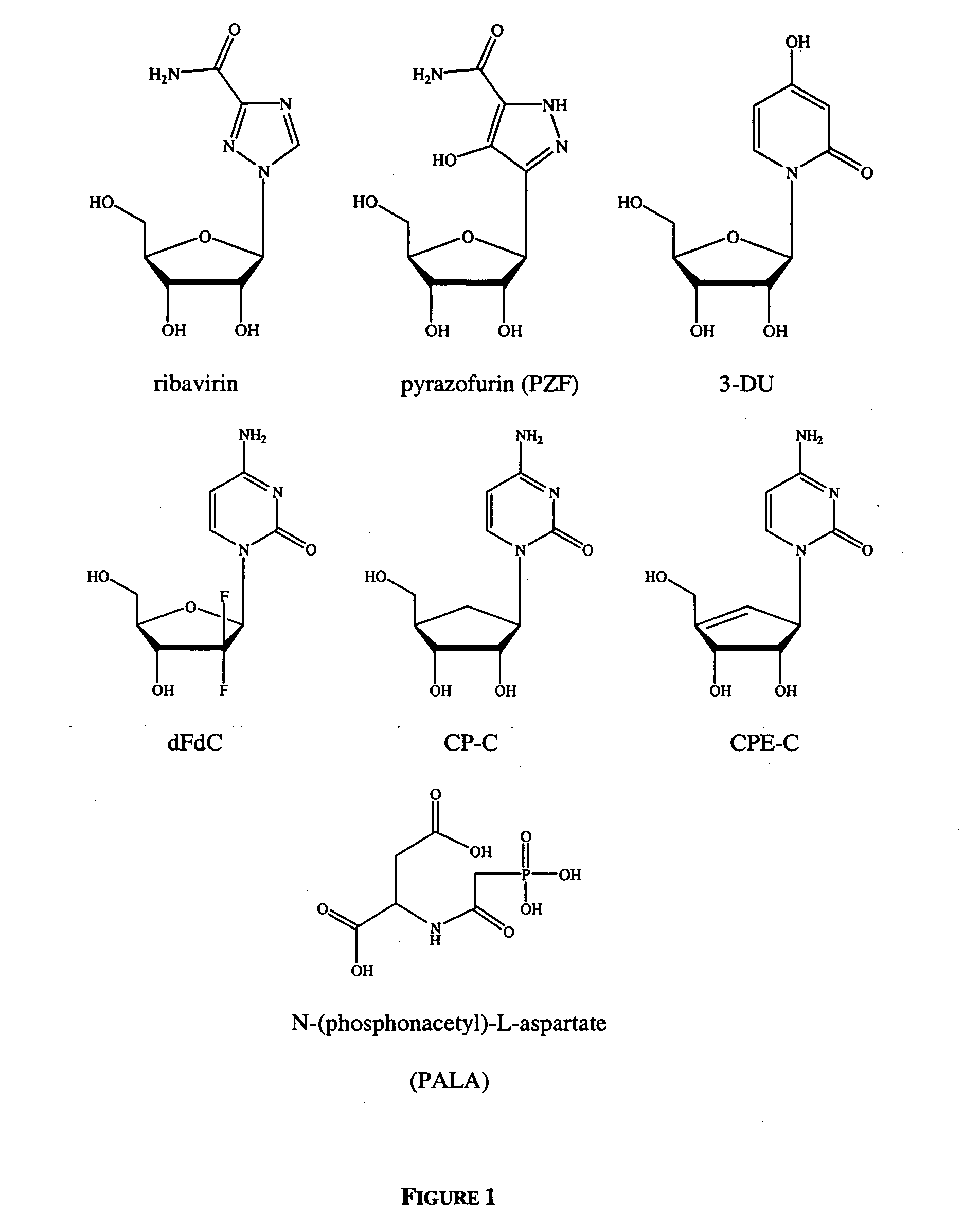
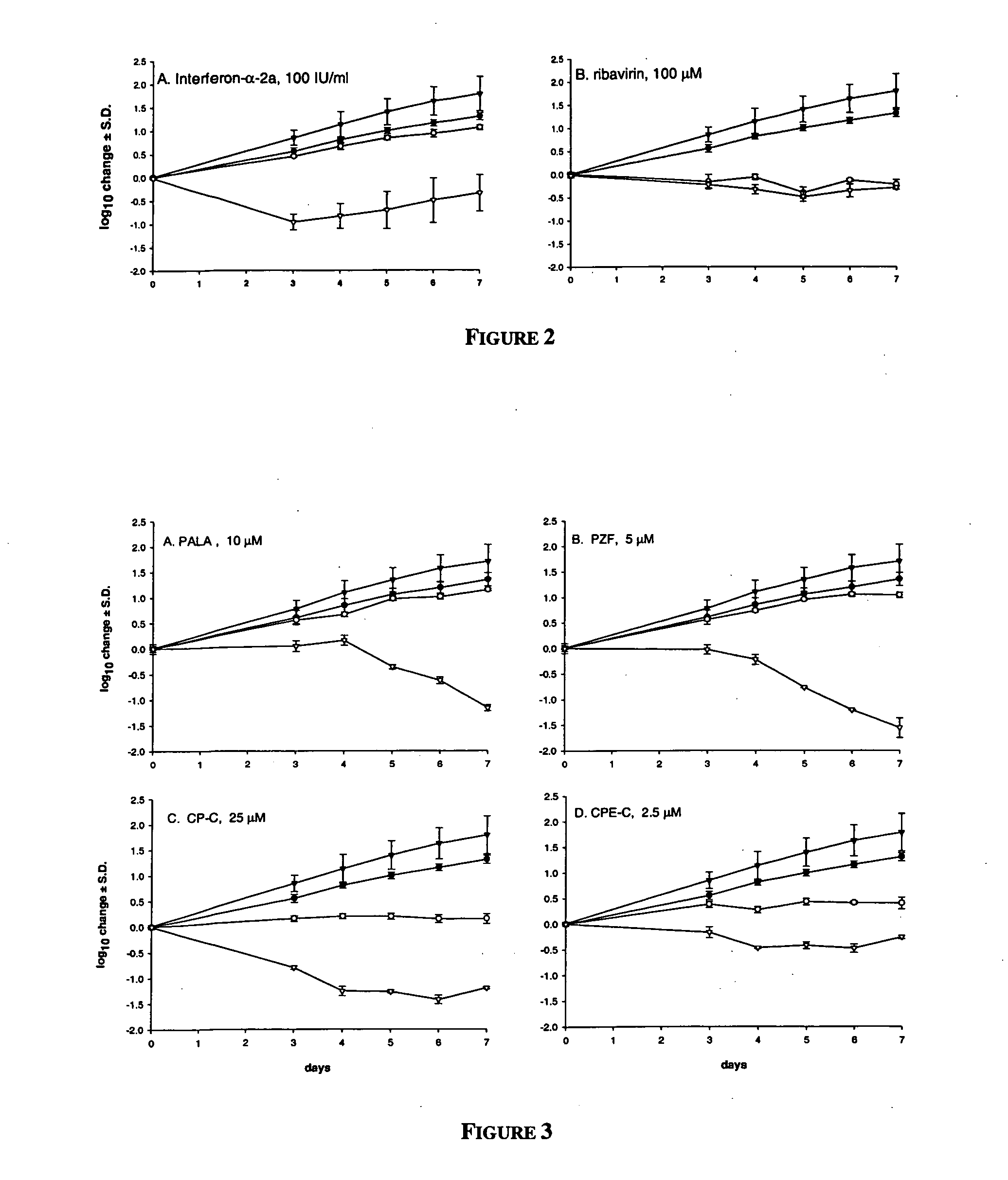
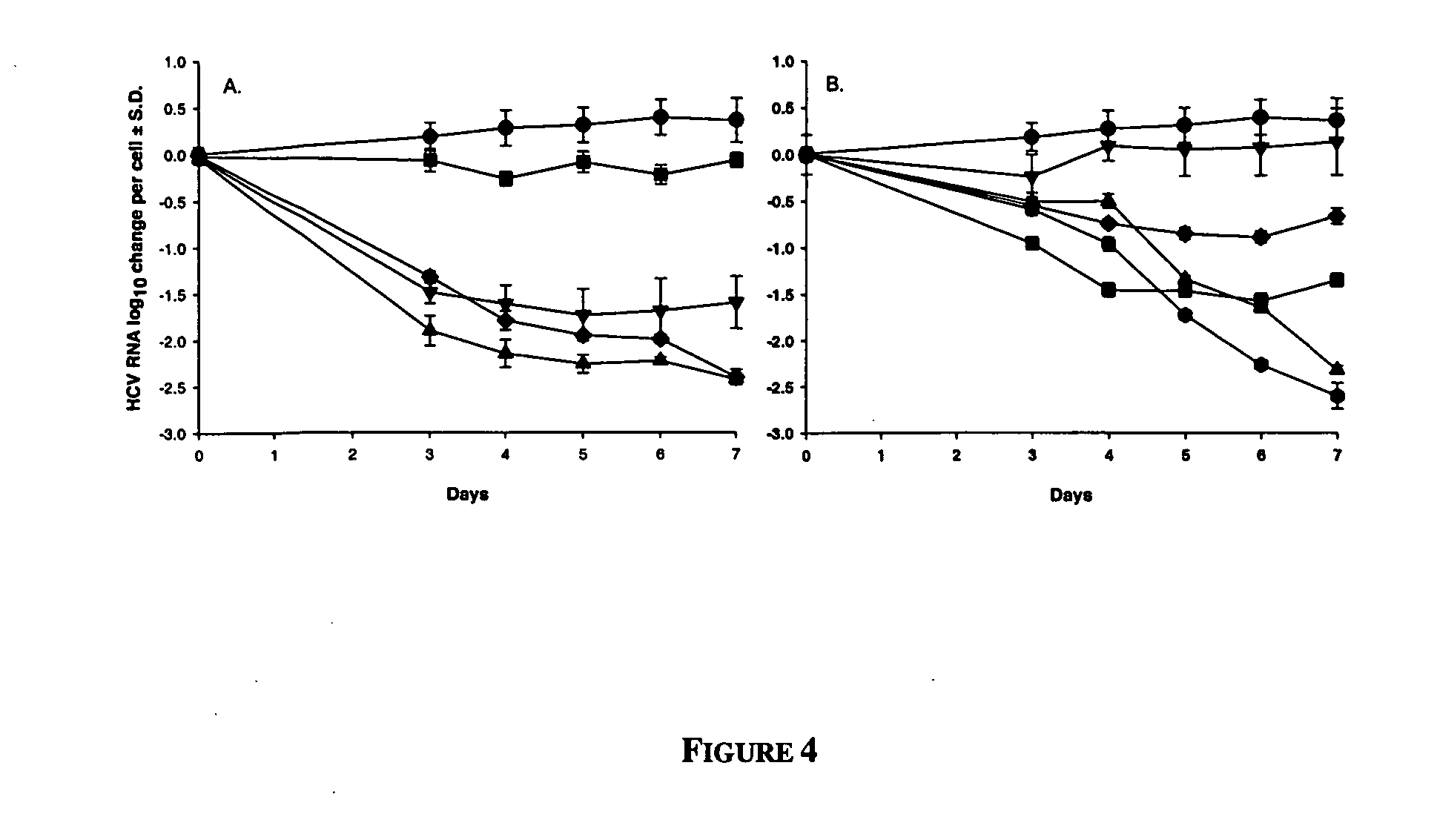
Dosing regimen for Flaviviridae therapy
An anti-hepatitis C agent which is an anti-metabolite to the host and cannot be administered on a daily or chronic basis as is usual in anti-viral therapy (referred to below as an “anti-HCV anti-metabolite”), can be administered using a traditional anti-cancer dosing regimen (for example via intravenous or parenteral injection), over a period of one, two, three, four, five, six, or seven days followed by cessation of therapy until rebound of the viral load is noted. This dosing regimen runs counter to conventional antiviral experience, wherein effective agents are usually administered over at least fourteen days of sustained therapy, and typically on an indefinite daily basis.
Owner:PHARMASSET
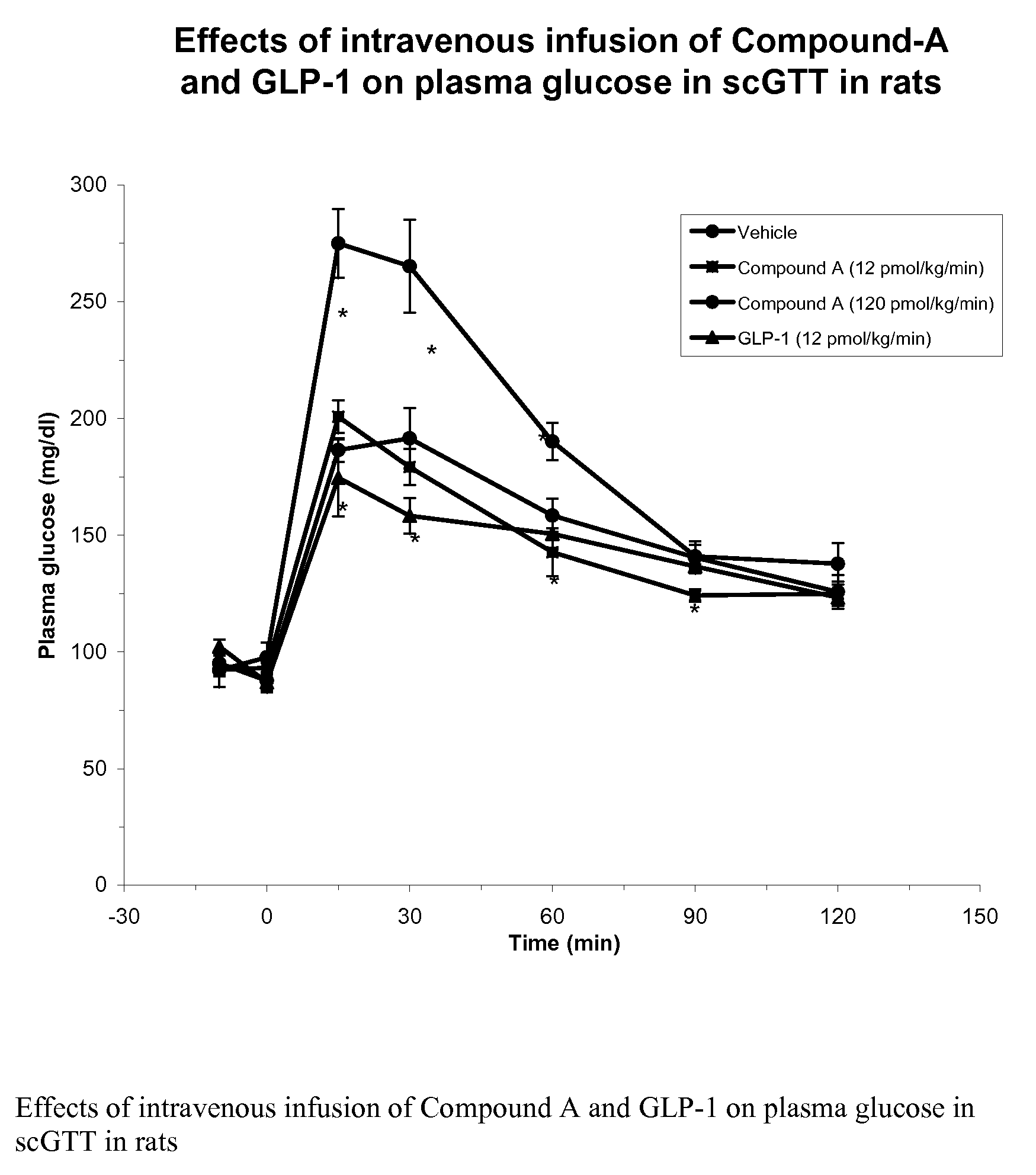
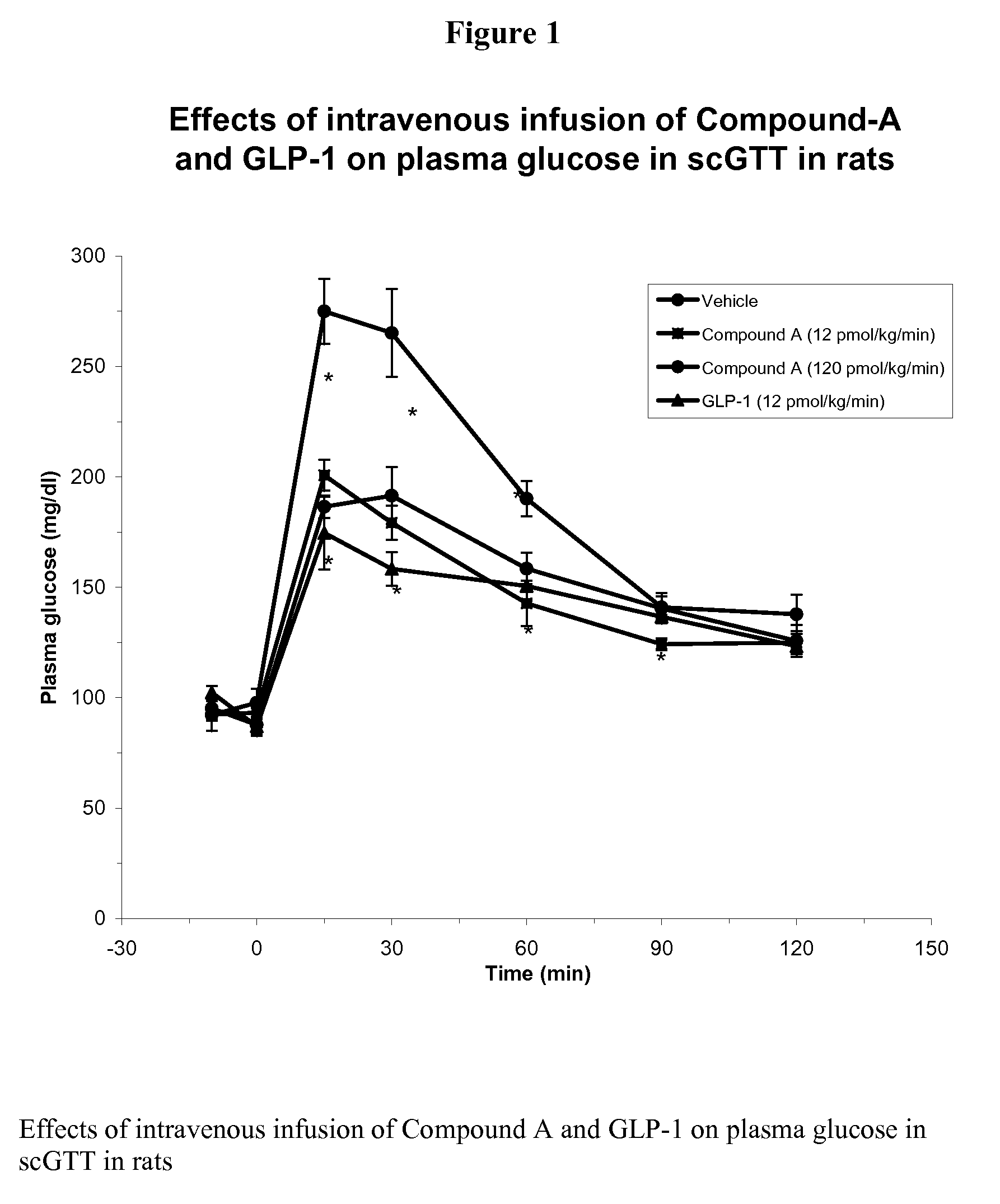
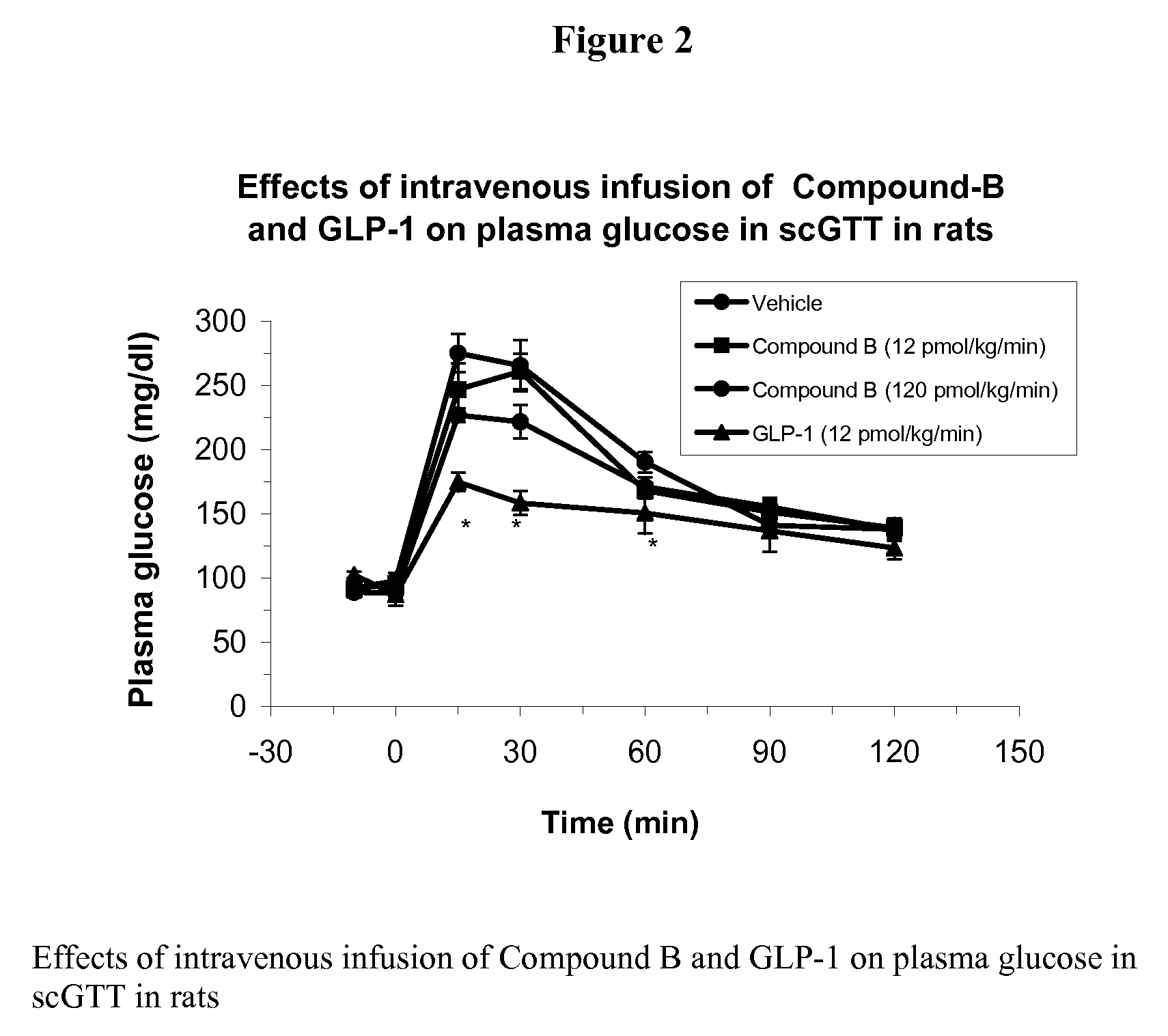
Human glucagon-like peptide-1 mimics and their use in the treatment of diabetes and related conditions
InactiveUS20070287670A1Improve the level ofSenses disorderNervous disorderGlucagon-like peptide-1Drug biological activity
The present invention provides novel human glucagon-like peptide-1 (GLP-1) peptide mimics that mimic the biological activity of the native GLP-1 peptide and thus are useful for the treatment or prevention of diseases or disorders associated with GLP activity. Further, the present invention provides novel, chemically modified peptides that not only stimulate insulin secretion in type II diabetics, but also produce other beneficial insulinotropic responses. These synthetic peptide GLP-1 mimics exhibit increased stability to proteolytic cleavage making them ideal therapeutic candidates for oral or parenteral administration.
Owner:BRISTOL MYERS SQUIBB CO
Selective opioid compounds
ActiveUS20090209569A1Reducing lipid permeability of drugReduce penetrationAntibacterial agentsBiocideDiseaseInterstitial cystitis
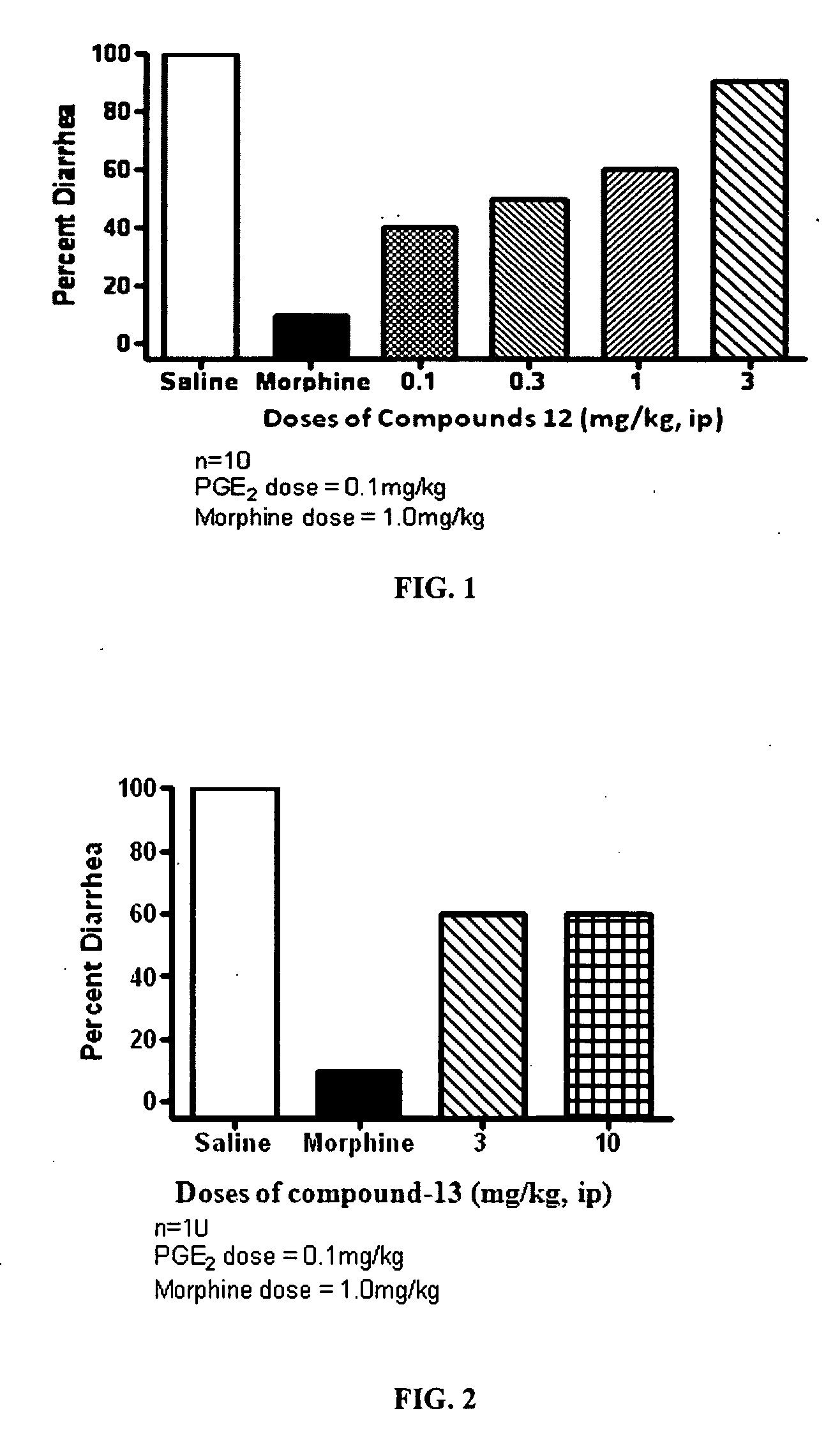
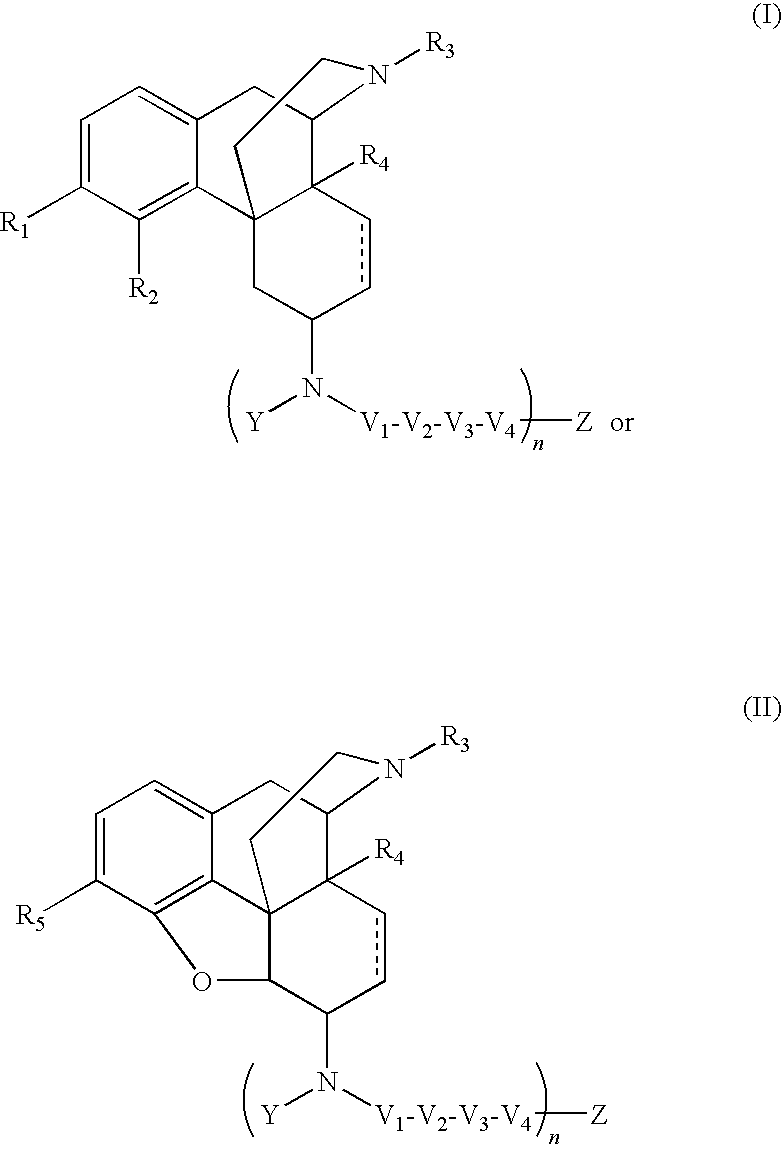
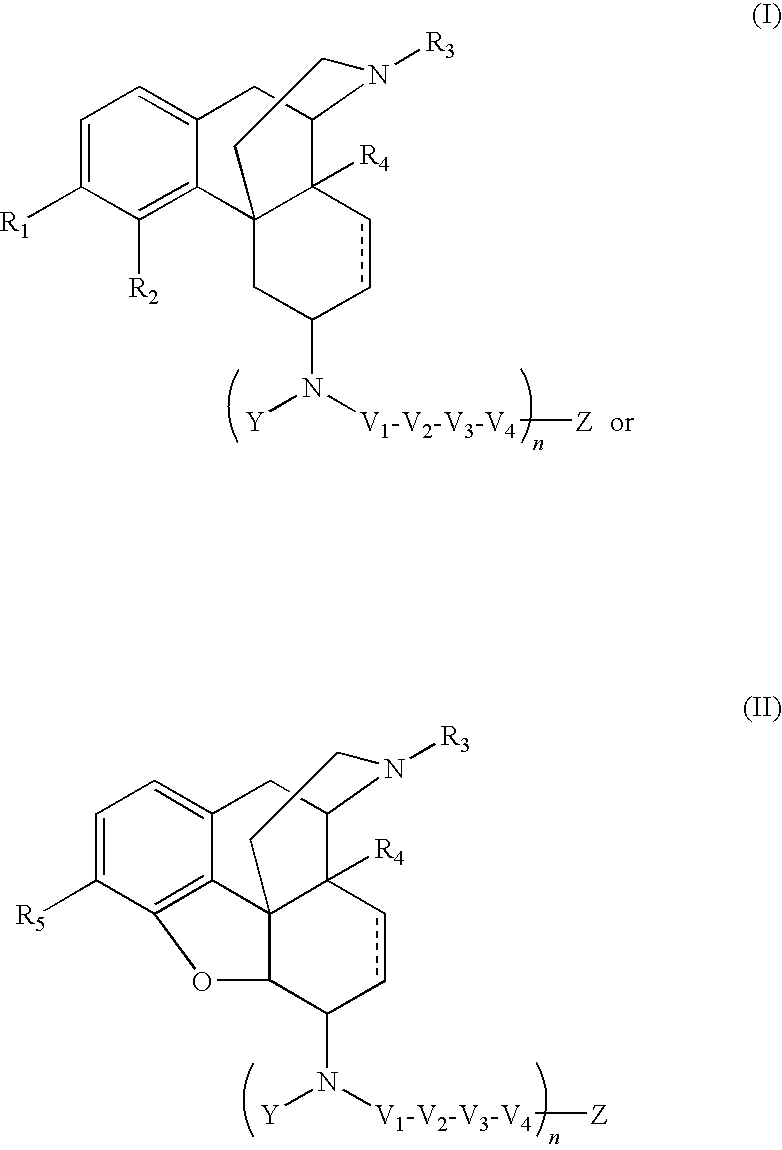
The present invention relates to compounds of Formula I or II, or pharmaceutically acceptable salts, esters, or prodrugs thereof:which relates to morphinan compounds useful as μ, δ, and / or κ receptor opioid compounds and pharmaceuticals containing same that may be useful for mediating analgesia, combating drug addiction, alcohol addiction, drug overdose, mental illness, bladder dysfunctions, neurogenic bladder, interstitial cystitis, urinary incontinence, premature ejaculation, inflammatory pain, peripherally mediated and neuropathic pain, cough, lung edema, diarrhea, cardiac disorders, cardioprotection, depression, and cognitive, respiratory, diarrhea, irritable bowel syndrome and gastro-intestinal disorders, immunomodulation, and anti-tumor agents.
Owner:ALKERMES INC
Treatment with agonists of toll-like receptors
InactiveUS20050163764A1Avoid damageMinimize complicationsBiocideOrganic active ingredientsToll-like receptorAgonist
Mammals are treated with agonists of bacterially-activated TLRs. The agonist are administered orally or mucosally. In one embodiment, the mammal treated is subject to a gastro-intestinal injury. The agonist can be administered prior to infliction of the gastro-intestinal injury, subsequent to infliction of the gastro-intestinal injury and concurrently with infliction of the gastro-intestinal injury. In another embodiment, the mammal is subject to tissue damage. The agonist is administered prior to the primary treatment, following the primary treatment or concurrently with the primary treatment.
Owner:YALE UNIV
Oral pharmaceutical composition
ActiveUS20100255087A1Improve solubilityImprove breathabilityOrganic active ingredientsNervous disorderSolid coreGastrointestinal tract
Owner:SUBLIMITY THERAPEUTICS LTD
Devices and methods for augmenting extragastric banding
InactiveUS20090093839A1Promoting tissue in-growthAltering abilitySurgeryDilatorsLower esophagusGastrointestinal device
The present invention is directed generically to a means for altering the ability of the mammalian body to absorb nutritive content from ingested foodstuffs, and more specifically to an apparatus and method of use for an endolumenal sleeve (referred to also as an “intragastrointestinal device” or “gastrointestinal device”) positioned in the mammalian gastrointestinal (GI) tract. A suitable endolumenal sleeve is comprised of an anchor element and an opening at a proximal end, an elongate lumen or hollow open-ended tube having a transverse dimension, and a distal orifice. Optionally, an exterior aspect of the elongate lumen may include additional modes of attachment to the tissues walls of the GI tract through the use of one or more means for promoting tissue in-growth. The endolumenal sleeve is retained in the GI tract such that a substantial fraction of the food and liquids passing through the GI tract is channeled into the proximal opening and through an interlumenal space defined within the interior space of the endolumenal sleeve. Within the endolumenal sleeve there may be one or more restrictive means to constrain, impede or otherwise control the operative flow of material through the device. An individual restrictive means can either be of a fixed geometry or such means may include one or more elements which are adjustable in nature or function. The elongate lumen of the endolumenal sleeve is formed of a polymer composition suitable for controlled ingress of biological secretions, egress of certain selected nutritional elements, and may comprise either a single tubular structure or a multi-section (i.e. articulated and / or multiple lumen) assembly. When the endolumenal sleeve is in situ within the mammalian gastro-intestinal system, ingested foodstuffs are conveyed from the proximal end to said distal orifice. In typical applications, the proximal end of the endolumenal sleeve is positioned within the physiological region extending from the lower esophagus to the duodenum and the distal orifice is positioned within the physiological region extending from the upper duodenum to the lower jejunum, though further extension into the lower intestine is possible. Through proper selection of position for the endolumenal sleeve proximal and distal ends, combined by selection of the composition used in the fabrication of the elongate lumen, it is possible to finitely control the degree of nutritive absorption performed by the gastrointestinal tract.
Owner:KELLEHER BRIAN
Myogenic cell transfer catheter and method
Catheters and methods for their use are presented that provide automated delivery of cells to structures in the body such as degenerative or weak muscle. The catheters contain one or more sensing components and a system for delivery of cells such as autologous myogenic cells. The sensing component(s) triggers automatic discharge of the cells upon detection of a body surface that needs repair. The discharge of cells may occur through an automated lancing mechanism that alleviates operator error from manual injection timing. During use, an operator can position the catheter end to a structure in need of cellular repair, and the device can automatically discharge cells at a location and time as determined by conditions sensed at the catheter end. A catheter and system is particularly useful for repair of degenerative and or weak heart muscle, such as that found after a myocardial infarct. The catheters and methods of their use also may be used for cellular repair of other interior body structures, particularly muscles such as bladder, intestine, stomach and diaphram. The catheters provide greater use of myogenic cell therapy for heart disease, while avoiding complications of open heart surgery.
Owner:LAW
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com