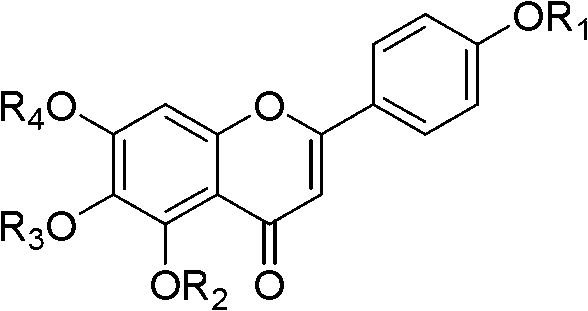
Scutellarin aglycone methylate product based on in-vivo metabolic mechanism as well as preparation method and application of scutellarin aglycone methylate product
A technology of scutellarin aglycon methyl and scutellarin aglycone, which is applied in the field of medicinal chemistry research and can solve the problem of low adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
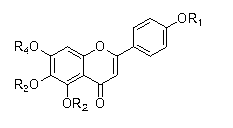
[0120] Example 15, Preparation of 6,7-trihydroxy-2-(4-methoxyphenyl)-4H-chromone (I-1)
[0121] (1) Preparation of 5,6,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromone (2)
[0122] Take scutellarin (1) (5g, 10.82mmol) and add to 3mol L -1 In 50mL of 90% concentrated sulfuric acid ethanol solution, 120°C with N 2 Protect the reaction for 48 hours, cool down after the reaction, pour the reaction solution into 8 times the amount of water, filter with suction, wash the filter cake with water until neutral, and dry at 50°C. The filter cake was repeatedly recrystallized with 80% and 50% ethanol to obtain 2 crude product. 2 The crude product was separated by silica gel column chromatography (dichloromethane:methanol=60:1) to obtain 790mg of the product. Yellow powder, yield 25.5%. 1 H NMR (300MHz, DMSO-d 6 )δ: 12.79(s,1H,5-OH);10.44(s,1H,7-OH);10.30(s,1H,4′-OH);8.71(s,1H,6-OH);7.90- 7.93(d,2H,J=8.8Hz,2′,6′-H);6.90-6.93(d,2H,J=8.8Hz,3′,5′-H);6.73(s,1H,8- H); 6.78(s,1H,3-H). ESI-MS...
Embodiment 26
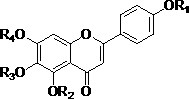
[0129] Example 26, Preparation of 7-dihydroxy-5-methoxy,-2-phenyl-4H-chromone (I-2)
[0130] (1) 6,7-dimethoxymethyleneoxy-5-hydroxy-2-(4′-methoxymethyleneoxyphenyl)-4H-1-benzopyran-4-one ( 5) Preparation
[0131] Take (2g, 7mmol) 2 and put it into 100mL of dry acetone, heat it to fully dissolve. Add (3.87g, 28mmol, 4equiv) K under ice bath and nitrogen protection 2 CO 3 , reacted for 30 minutes under ice bath and nitrogen protection, then added (2.39 mL, 31.5 mmol, 4.5 equiv) MOMCl, reacted at room temperature for 1 hour, and then refluxed for 4 hours. The reaction solution was suction filtered, the filter cake was washed with acetone, and the combined filtrates were concentrated. Separation and purification by silica gel column chromatography, eluent petroleum ether: ethyl acetate (4:1). 2.31 g of (5) was obtained as a yellow solid with a yield of 79%. 1 H NMR 300MHz, DMSO-d 6 )δ: 12.93(s,1H,5-OH);8.05-8.08(d,2H,J=8.7Hz,2′,6′-H);7.14-7.17(d,2H,J=8.7Hz,3 ′,5′-H);7.09(...
Embodiment 35
[0136] Example 35, Preparation of 7-dihydroxy-6-methoxy-2-phenyl-4H-chromone (I-3)
[0137](1) 5-Hydroxy-2-(4′-benzyloxyphenyl)-4H-1-{2,2-diphenyl-[1,3]-dioxo[5,4-d]benzene Preparation of}pyran-4-one (7)
[0138] Take (200mg, 0.44mmol) 3 into 10mL of DMF, add (107mg, 0.78mmol, 1.75equiv) K 2 CO 3 After reacting at room temperature for 30 minutes, BnBr (0.08 mL, 0.66 mmol, 1.5 equiv) was added and reacted at room temperature for 6 hours. The reaction solution was diluted with 80mL water, extracted with 20mL×4 ethyl acetate, washed three times with water, anhydrous Na 2 SO 4 dry. Filtrate, recover the solvent, separate and purify by silica gel column chromatography, eluent petroleum ether: ethyl acetate (8:1). 197 mg of yellow solid 7 was obtained, with a yield of 83%. 1 H NMR (300MHz, DMSO-d 6 )δ: 13.10 (s, 1H, 5-OH); 10.40 (s, 1H, 4′-OH); 8.03-8.05 (d, 2H, J=8.7Hz, 2′, 6′-H); 7.55- 7.61;7.46-7.48and 7.32-7.43(each m,15H,-Ph);7.21-7.24(d,2H,J=8.7Hz,3′,5′-H);7.08(s,1H,8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com