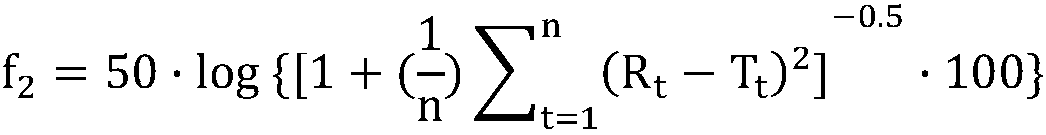Desvenlafaxine hydrochloride pharmaceutical composition and preparation method thereof
A technology of desvenlafaxine and composition, which is applied in the directions of drug combination, pharmaceutical formula, sugar-coated pill, etc., can solve the problems of inability to coat, uneven particle size, low hardness of plain tablet, etc., and achieves overcoming uneven addition , The production process is easy to operate, and the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
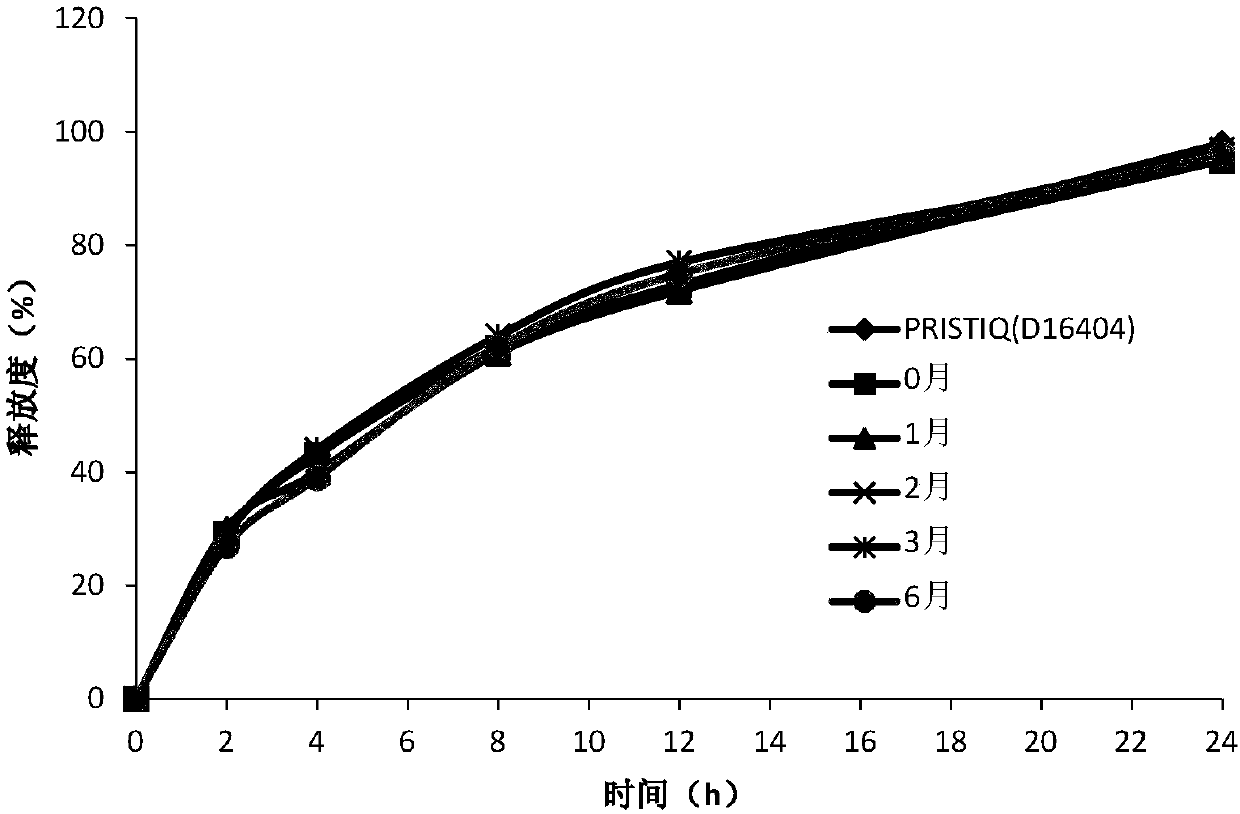
[0041] Six experiments were designed to investigate the effect of the concentration of wetting agent on the state of the obtained granules, the difficulty of sieving and the bulk density of the dried granules, and the ratio of ethanol / water was determined.
[0042] (a) Prescription of plain tablets
[0043] Table 1 Unit: mg
[0044]
[0045] (b) Prepare above-mentioned prescription according to A, preparation method, investigate the degree of difficulty of the granulation process in the preparation process, each test mainly takes the uniformity of granules, the degree of difficulty during granulation, and the bulk density of dry granules as evaluation indicators Make an evaluation.
[0046] Table 2: Evaluation Results
[0047]
[0048]
[0049] As shown in Table 2, when the concentration of ethanol is 60% to 95% or absolute ethanol, the obtained particles are uniform, easy to sieve, and the bulk density of the material is moderate, which is beneficial to the filling...
Embodiment 7-10
[0051] 4 experiments were designed to investigate the influence of different dosages of hypromellose on dissolution, and the dissolution characteristics were determined by f 2 factors are evaluated.
[0052] (a) Prescription of plain tablets
[0053] Table 3 Unit: mg
[0054]
[0055] (b) prepare above-mentioned prescription according to A, preparation method, f 2 Factors are used to evaluate the evaluation indicators.
[0056] Table 4: Evaluation Results (Unit: %)
[0057]
[0058]
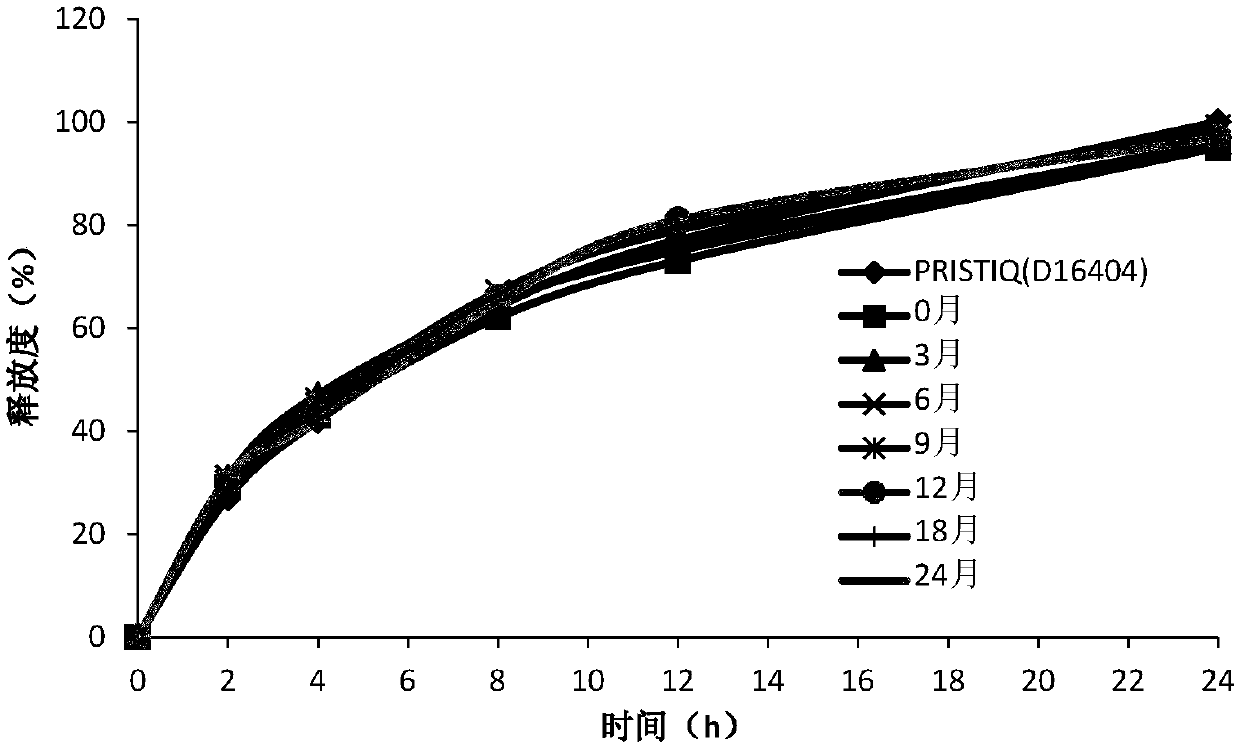
[0059] As shown in Table 4, the release curves of hypromellose at 50%-80% dosage were similar. When the amount of hypromellose is less than 50%, the release curve is not similar; when the amount of hypromellose is greater than 80%, it is difficult to prepare tablets according to A, the preparation method.
Embodiment 11-14
[0061] 4 experiments were designed to investigate the influence of different dosages of hypromellose on dissolution, and the dissolution characteristics were determined by f 2 factors are evaluated.
[0062] (a) Prescription of plain tablets
[0063] Table 5 Unit: mg
[0064]
[0065] (b) prepare above-mentioned prescription according to A, preparation method, f 2 Factors are used to evaluate the evaluation indicators.
[0066] Table 6: Evaluation Results (Unit: %)
[0067]
[0068] As shown in Table 6, the release curves of hypromellose at 60%-80% dosage are similar.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Depth | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com