Atorvastatin calcium tablet and preparation method thereof
A technology of atorvastatin calcium and calcium carbonate, which is applied in the direction of pharmaceutical formulations, medical preparations of non-active ingredients, pill delivery, etc., can solve the problems of atorvastatin calcium instability, affecting clinical efficacy, and poor water solubility. Achieve the effects of easy industrialization, short disintegration time and fast dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
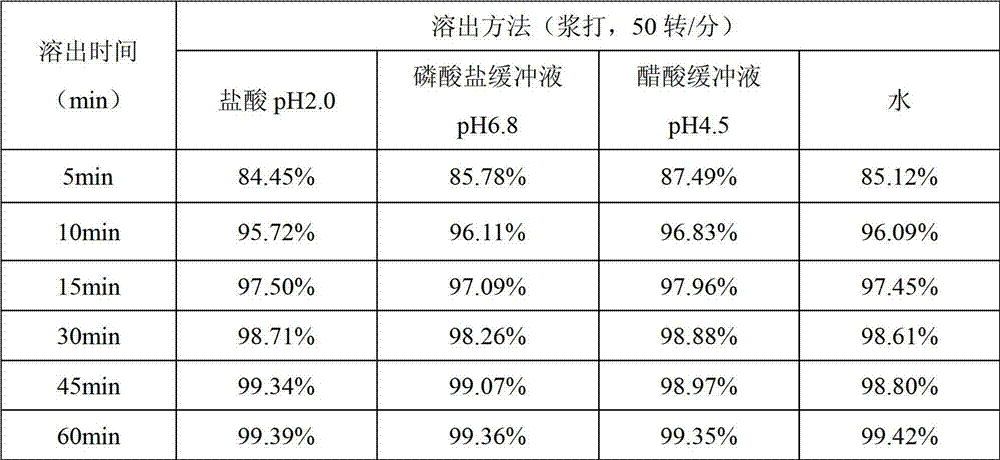
Examples
Embodiment 1
[0021] The atorvastatin calcium tablet of the present embodiment comprises the following components by mass: 7.22 parts of atorvastatin calcium, 22.01 parts of calcium carbonate, 21.87 parts of lactose, 40.67 parts of microcrystalline cellulose, and croscarmellose 6 parts of sodium cellulose, 1.33 parts of hydroxypropyl cellulose, 0.4 parts of polysorbate-800, and 0.5 parts of magnesium stearate.
[0022] The preparation method of the atorvastatin calcium tablet provided in this embodiment (tablet core weight 150mg, taking 1000 tablets as an example), comprises the following steps:
[0023] 1) Pass atorvastatin calcium through a 200-mesh sieve, pass lactose, microcrystalline cellulose, calcium carbonate, and croscarmellose sodium through a 80-mesh sieve, and set aside;
[0024] 2) Heat 10g of purified water to 50°C, add 0.6g of polysorbate-80, stir to dissolve completely, add 2.0g of hydroxypropyl cellulose (HPC-L), stir well, add 51g of purified water, and swell 4h, as an ad...
Embodiment 2
[0033] The atorvastatin calcium tablet of the present embodiment comprises the following components by mass: 7.22 parts of atorvastatin calcium, 22.01 parts of calcium carbonate, 21.87 parts of lactose, 40.67 parts of microcrystalline cellulose, and croscarmellose 6 parts of sodium cellulose, 1.33 parts of hydroxypropyl cellulose, 0.4 parts of polysorbate-800, and 0.5 parts of magnesium stearate.
[0034] The preparation method of the atorvastatin calcium tablet provided in this embodiment (tablet core weight 150mg, taking 1000 tablets as an example), comprises the following steps:
[0035] 1) Pass atorvastatin calcium through a 200-mesh sieve, pass lactose, microcrystalline cellulose, calcium carbonate, and croscarmellose sodium through a 80-mesh sieve, and set aside;
[0036] 2) Heat 12g of purified water to 50°C, add 0.6g of polysorbate-80, stir to dissolve completely, add 2.0g of hydroxypropyl cellulose (HPC-L), stir well, add 50g of purified water, and swell 5h, as an ad...
Embodiment 3
[0045] The atorvastatin calcium tablet of the present embodiment comprises the following components by mass: 7.22 parts of atorvastatin calcium, 22.01 parts of calcium carbonate, 21.87 parts of lactose, 40.67 parts of microcrystalline cellulose, and croscarmellose 6 parts of sodium cellulose, 1.33 parts of hydroxypropyl cellulose, 0.4 parts of polysorbate-800, and 0.5 parts of magnesium stearate.
[0046] The preparation method of the atorvastatin calcium tablet provided in this embodiment (tablet core weight 150mg, taking 1000 tablets as an example), comprises the following steps:
[0047] 1) Pass atorvastatin calcium through a 200-mesh sieve, pass lactose, microcrystalline cellulose, calcium carbonate, and croscarmellose sodium through a 80-mesh sieve, and set aside;
[0048] 2) Heat 15g of purified water to 50°C, add 0.6g of polysorbate-80, stir to dissolve completely, add 2.0g of hydroxypropyl cellulose (HPC-L), stir well, add 55g of purified water, and swell 6h, as an ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com