Preparation method of paclitaxel injection
A paclitaxel and injection technology, applied in the field of pharmaceutical preparations, can solve problems such as low pH and stability, and achieve the effects of increasing compliance and safety, reducing adverse reactions, and increasing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018]
[0019] 1) Weigh 375g of cation exchange resin, fill it in a clean glass tube packed column, moisten it with 500ml water for injection, press filter to remove excess water, then rinse with 500ml of 4% sodium hydroxide solution in sequence, and then use 500ml of injection Rinse the resin-packed column with water and 1000ml of 5% hydrochloric acid solution, cycle twice, and finally rinse with 500ml of water for injection and press filter to remove excess water;
[0020] 2) Take out the resin in the packed column, place it in an oven at 60°C and dry it to a constant weight, then soak the dried resin in an equal volume of absolute ethanol for 30 minutes, and dry it;
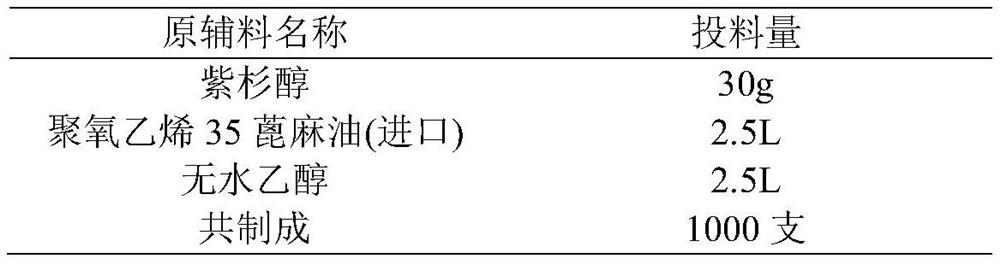
[0021] 3) Stir and mix 2.5L polyoxyethylene 35 castor oil (imported from BASF) and 2.5L absolute ethanol, add resin, and stir at about 20°C for 2 hours. Stirring was stopped, the mixed solution was allowed to stand for stratification, and the supernatant was filtered through a 1.2 μm polytetrafluoroethylen...
Embodiment 2
[0028]
[0029] 1) Weigh 375g of cation exchange resin, fill it in a clean glass tube packed column, moisten it with 500ml water for injection, press filter to remove excess water, then rinse with 500ml of 4% sodium hydroxide solution in sequence, and then use 500ml of injection Rinse the resin-packed column with water and 1000ml of 5% hydrochloric acid solution, cycle 4 times, and finally rinse with 500ml of water for injection and press filter to remove excess water;
[0030] 2) Take out the resin in the packed column, place it in an oven at 60°C and dry it to a constant weight, then soak the dried resin in an equal volume of absolute ethanol for 30 minutes, and dry it;
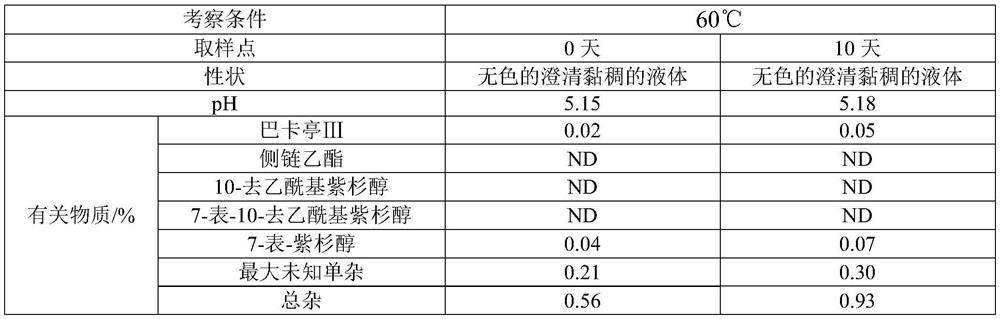
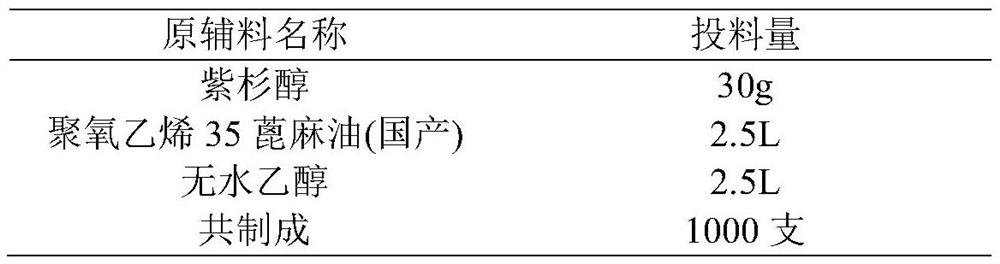
[0031] 3) Stir and mix 2.5L polyoxyethylene 35 castor oil and 2.5L absolute ethanol, add resin, and stir at about 25°C for 4 hours. Stirring was stopped, the mixed solution was allowed to stand for stratification, and the supernatant was filtered through a 0.22 μm polytetrafluoroethylene filter element; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com