Lurasidone hydrochloride oral preparation and preparing method of lurasidone hydrochloride oral preparation
A technology of lurasidone hydrochloride and oral preparations, which is applied in the field of medicine, can solve the problems of large static electricity of raw materials, inability to obtain sufficient immediate release and solubility, and large loss, and achieve small intra-batch differences, small losses, and high yields Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The preparation method of lurasidone hydrochloride tablet in following each embodiment is as follows:
[0036] (1) Weigh the prescription amount of lurasidone hydrochloride raw material and optional silicon dioxide, mix them, pulverize and control the particle size to 0.1-20 μm, and set aside;
[0037] (2) Preparation of an aqueous solution of a water-soluble polymer binder: a water-soluble polymer binder was dissolved in pure water. The amount of water-soluble polymer binder is for example 1 to 20wt% of pure water weight, preferably 2 to 8wt%, the water-soluble polymer binder that can be used is for example hypromellose, polyvinylpyrrolidone or polyvinyl alcohol .
[0038] (3) Preparation of granules containing lurasidone hydrochloride: adding a mixture containing lurasidone hydrochloride and optional silicon dioxide, mannitol, lactose, low-substituted hydroxypropyl cellulose, Croscarmellose Sodium, mix well. Add the aqueous binder solution prepared in (2) to make s...
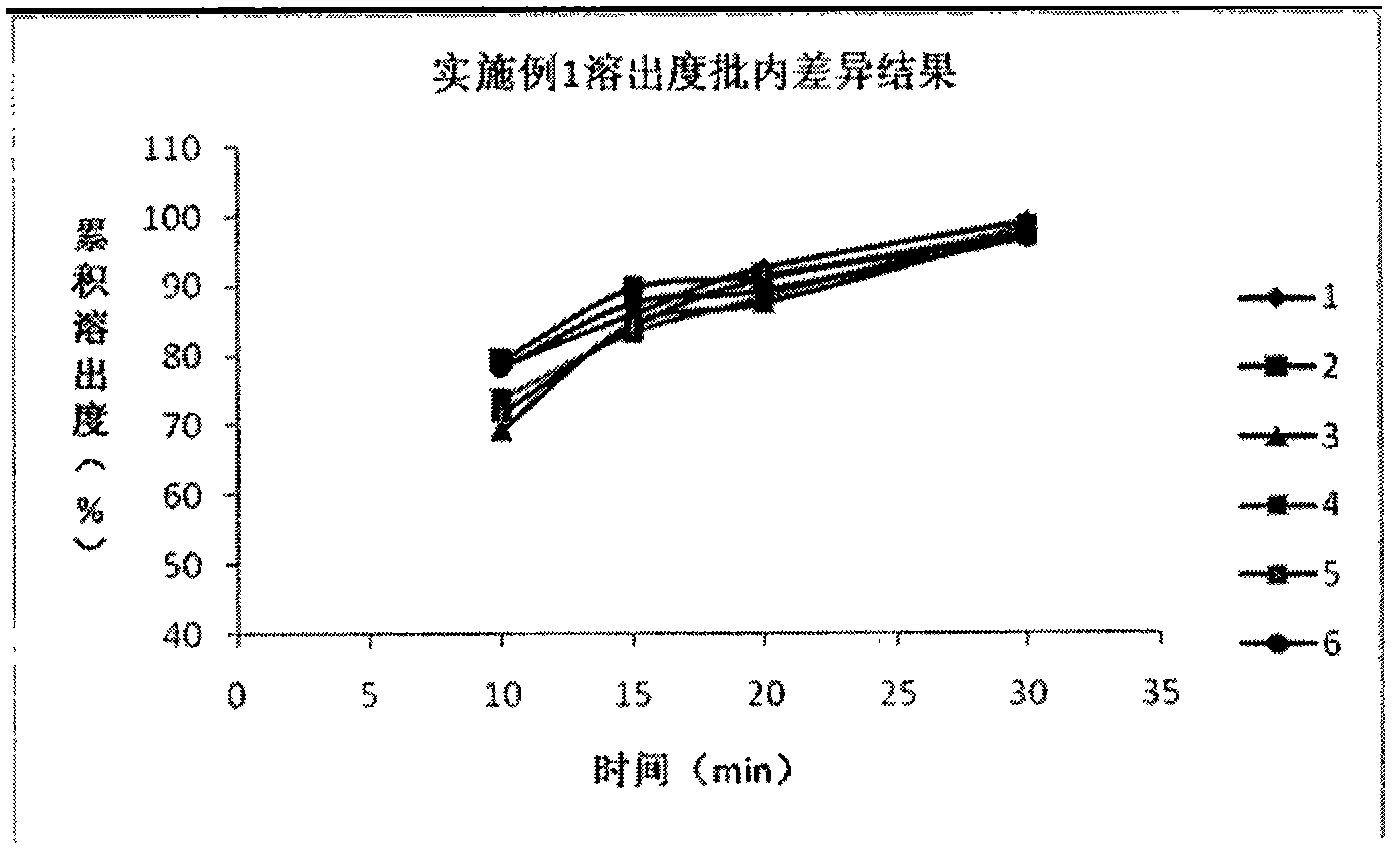
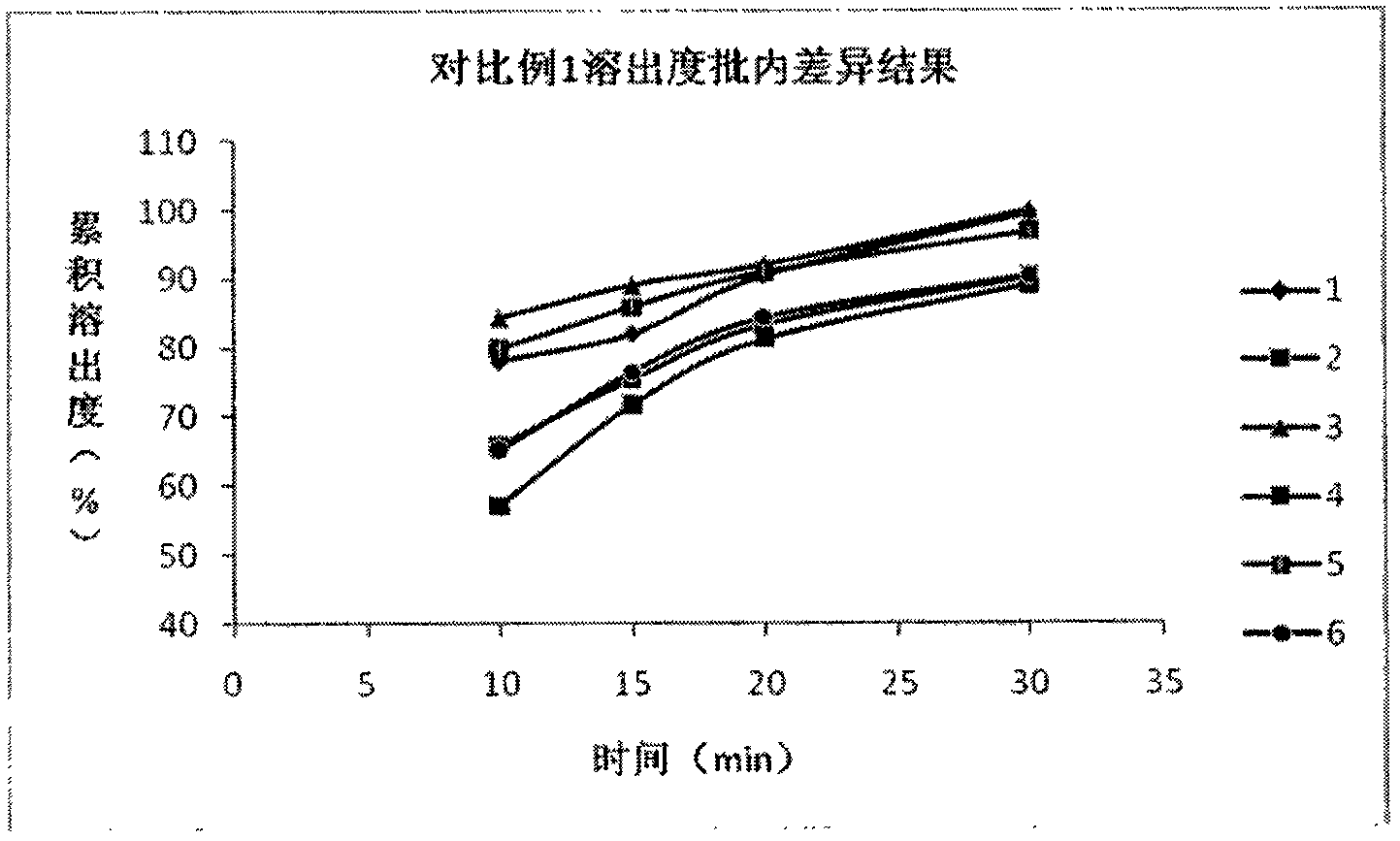
Embodiment 1 and comparative example 1 and 2
[0044] (1) embodiment 1 and comparative example 1 and 2 raw materials and proportioning ratio as shown in table 1:
[0045] Table 1. Embodiment 1 and comparative examples 1 and 2 raw materials and proportioning (batch: 1000)
[0046]
Example 1
Comparative example 1
Comparative example 2
80.0g
80.0g
80.0g
116.0g
116.0g
144.0g
58.0g
58.0g
72.0g
[0047] Low-substituted hydroxypropyl cellulose (hydroxypropyl content%)
42.0g(11%)
50.0g(11%)
-
8.0g
-
8.0g
silica
6.0g
6.0g
6.0g
7.0g
7.0g
7.0g
3.2g
3.2g
3.2g
Opadry Film Coating Premix
8.3g
8.3g
8.3g
[0048] (2), test
[0049] (2.1) Dissolution test: According to the research results of the dissolution curve of the reference prepar...
Embodiment 1-4
[0065] (1) embodiment 1-4 raw material and proportioning ratio as shown in table 6:
[0066] Table 6. Embodiment 1-4 raw material and proportioning (batch: 1000 pieces)
[0067]
[0068]
[0069] (2), test
[0070] Embodiment 1-4 dissolution rate result is as table 8
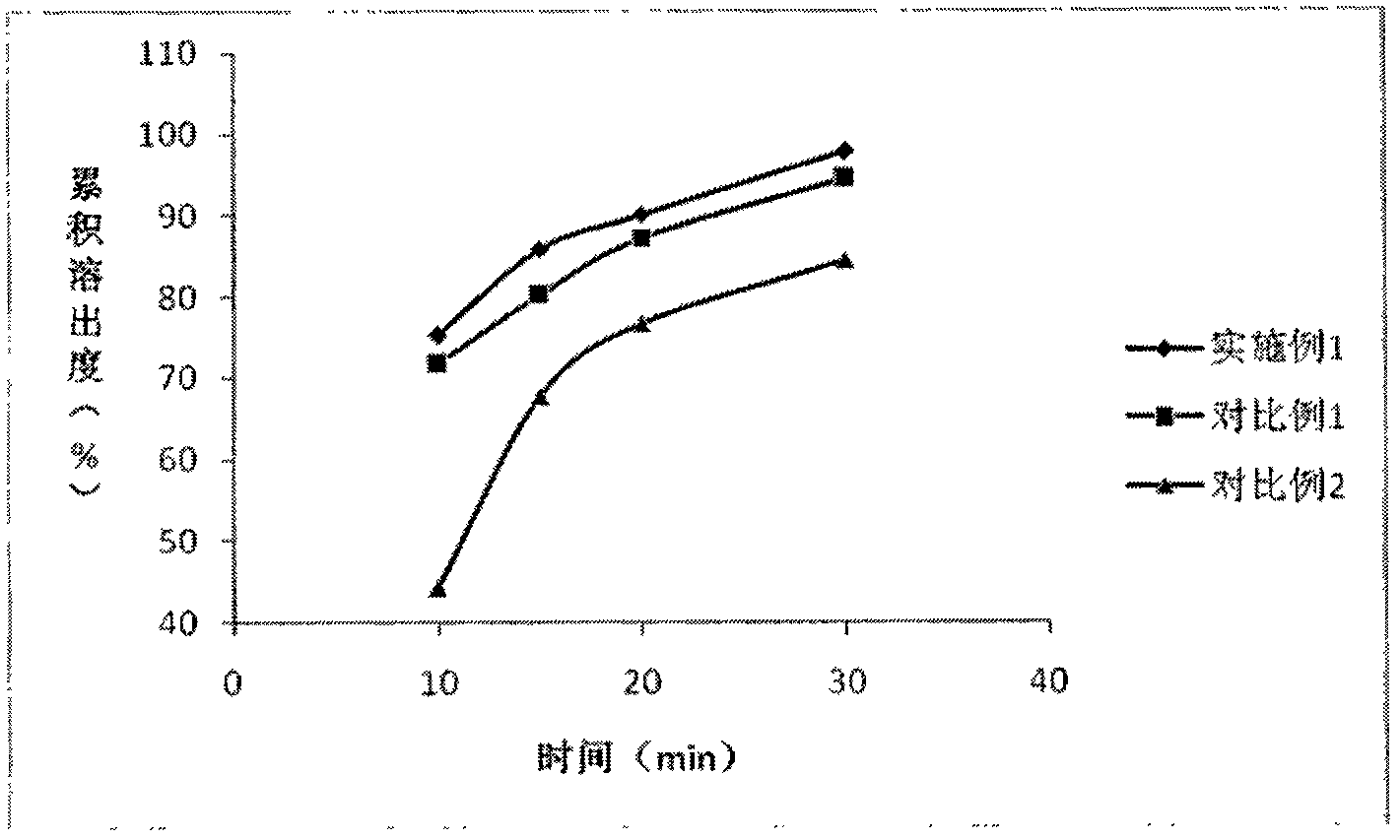
[0071] Table 7 Dissolution test results
[0072]
[0073] As shown in Table 7, the f2 values in Examples 2-4 show similarities to Example 1. That is, as shown in Table 7 and Figure 4 As shown, in Examples 1-4, the f2 value representing the similarity of dissolution properties is in the range of 50≤f2≤100, and preparations with similar dissolution properties are obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com