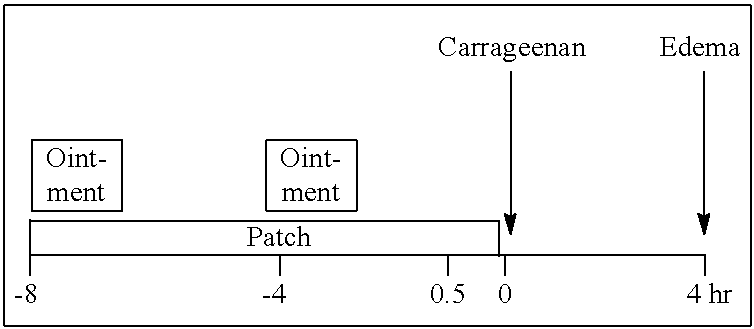
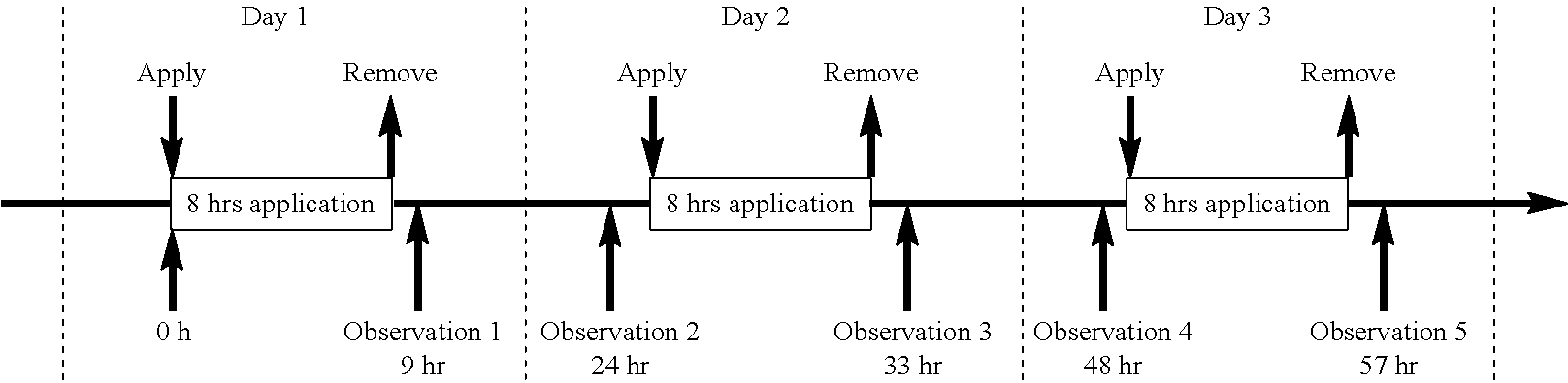
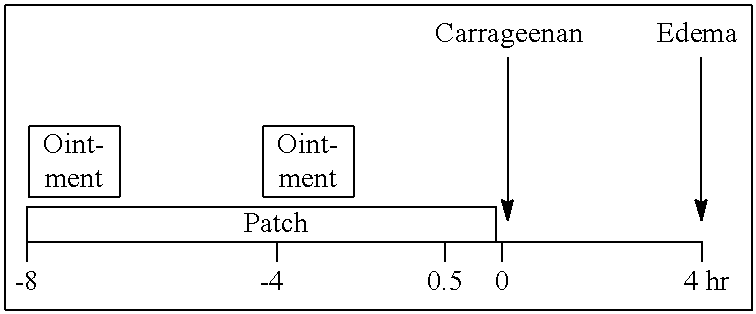
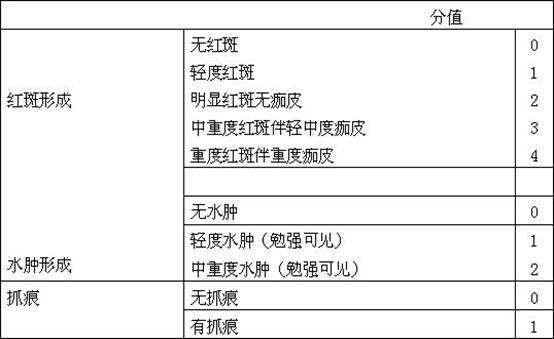
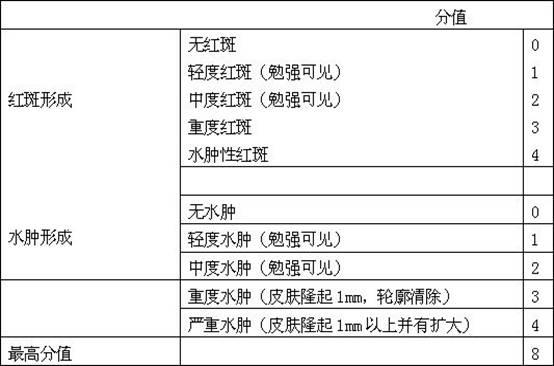
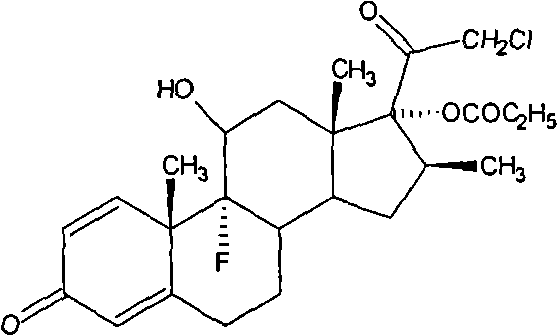
Patents
Literature
134 results about "Clobetasol propionate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
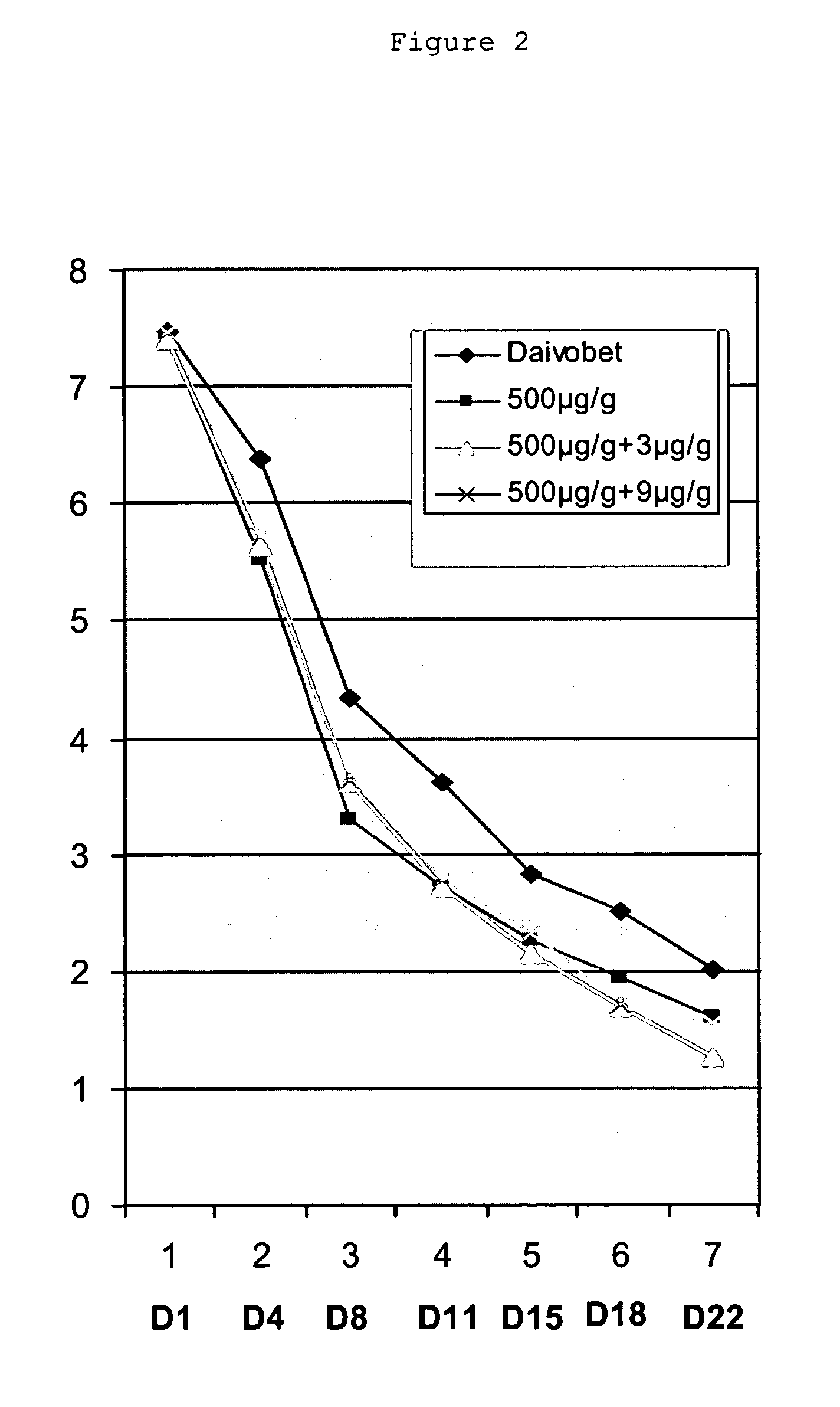
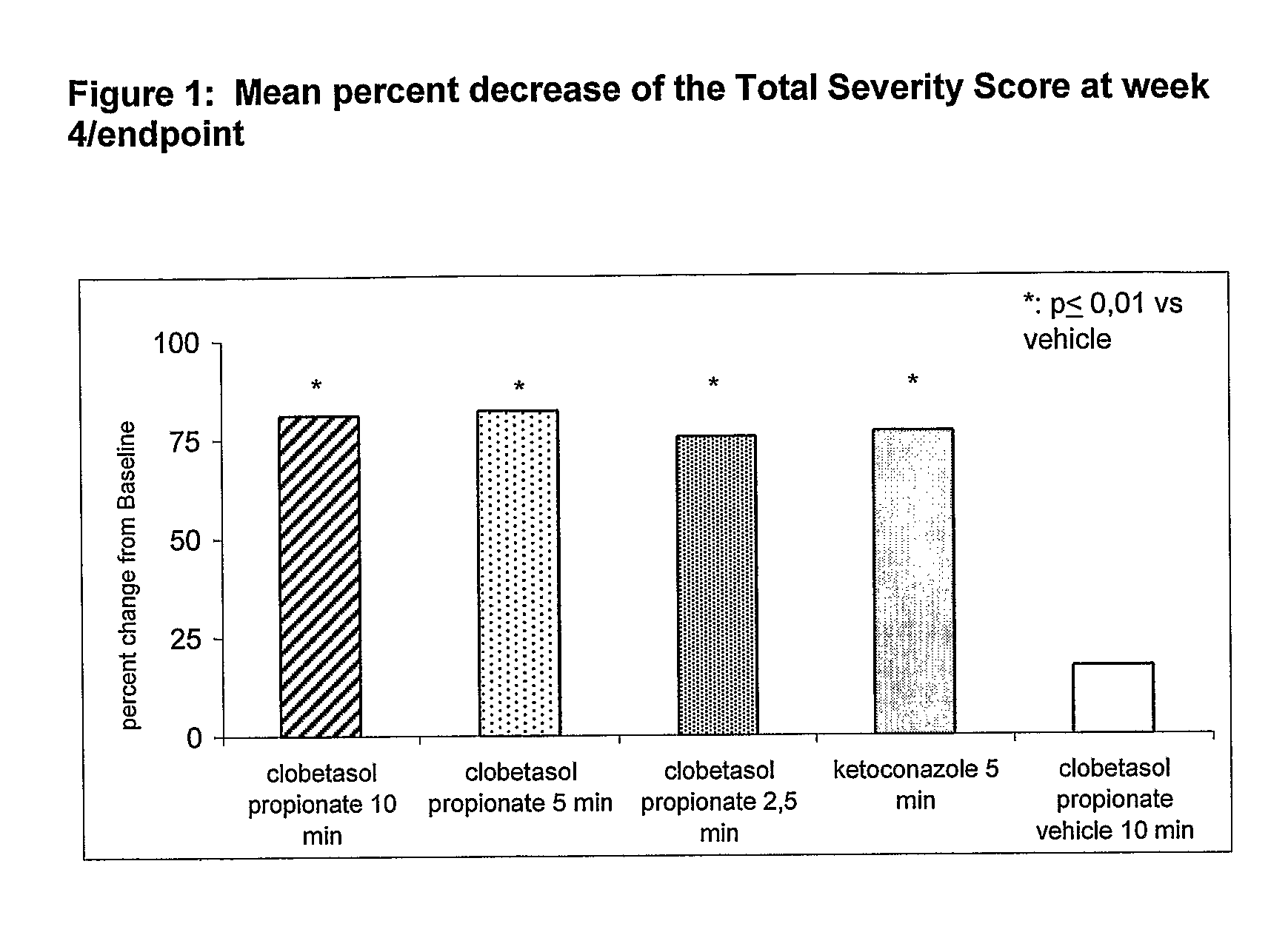
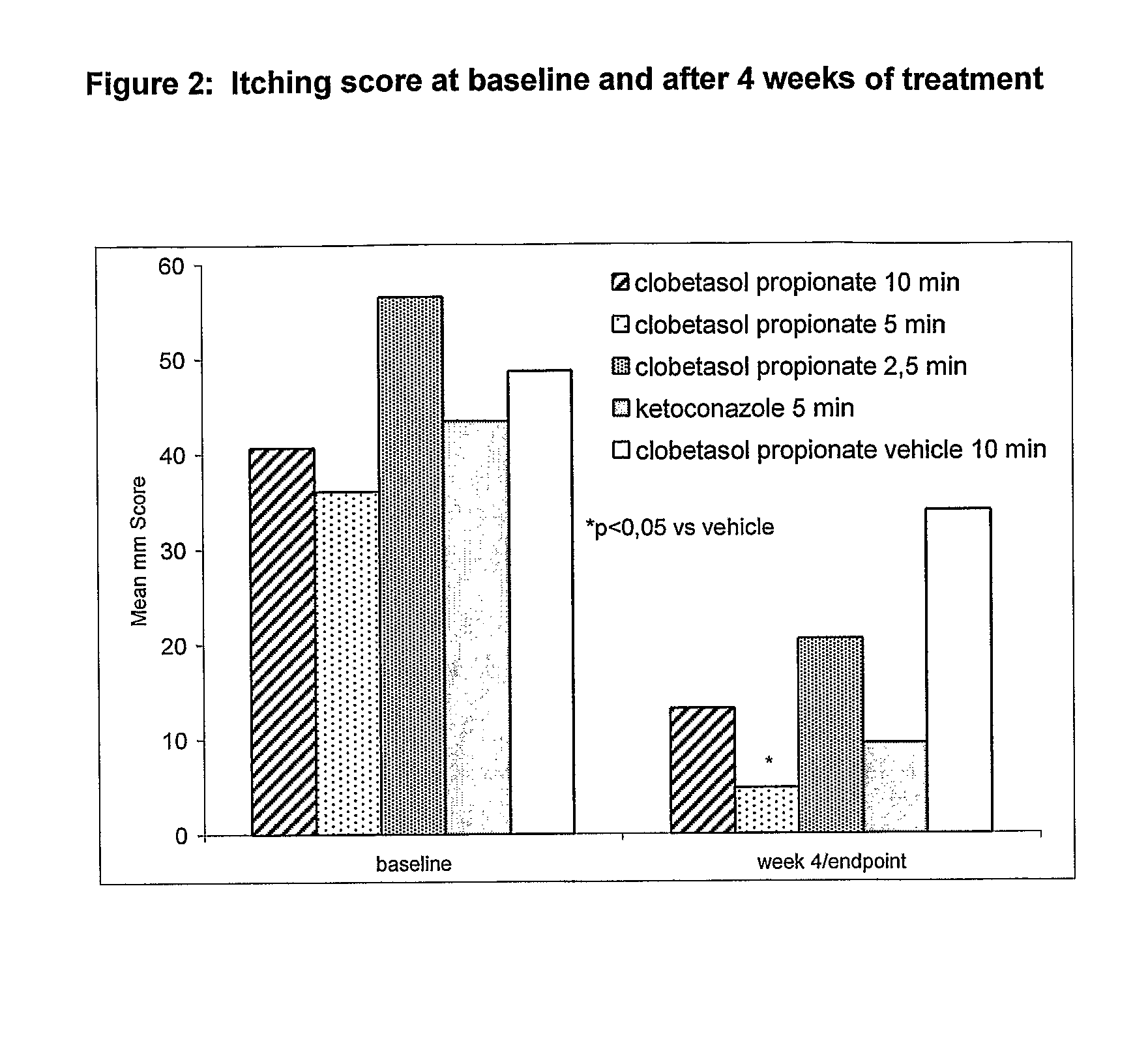
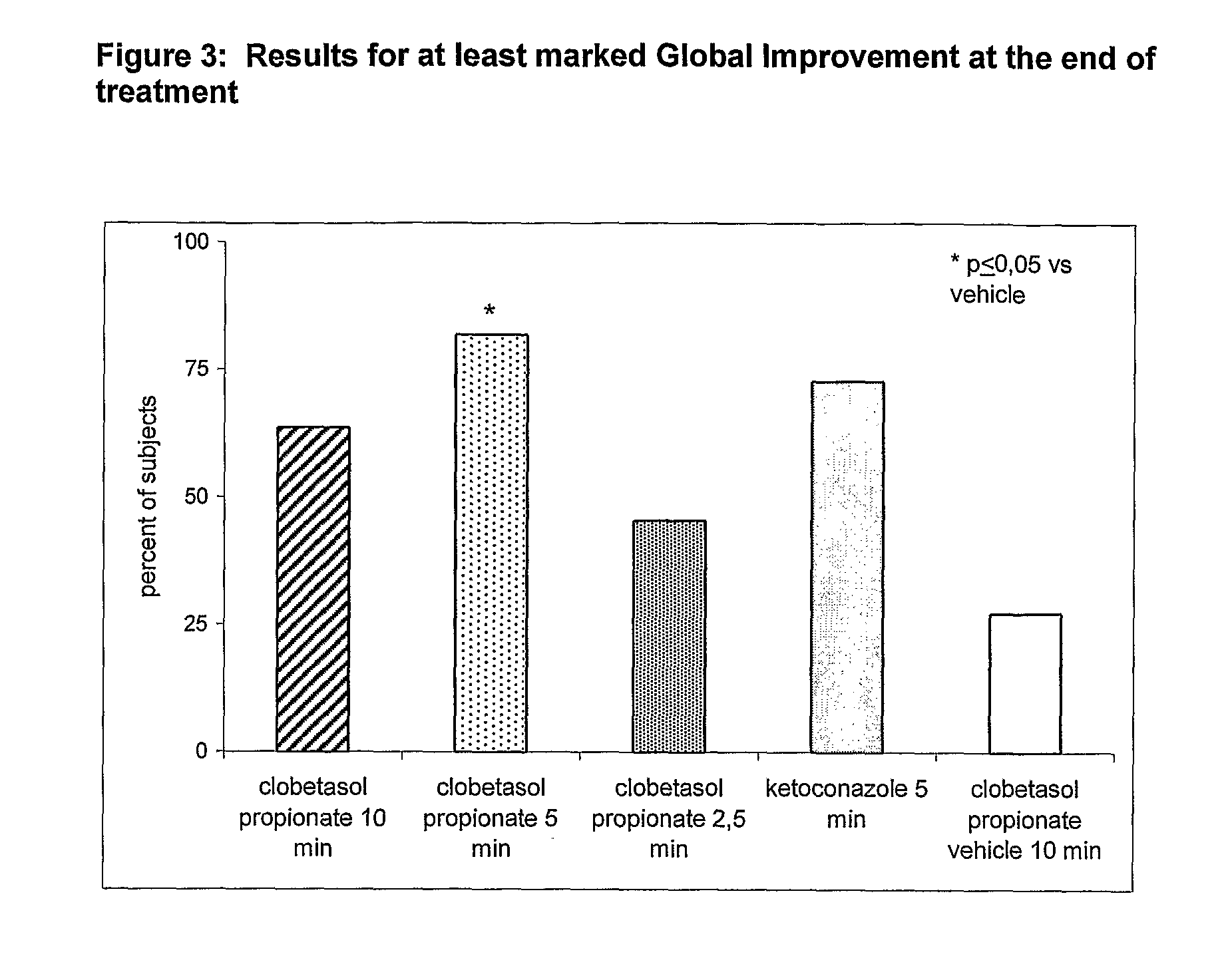
Clobetasol is used in adults to treat moderate to severe plaque psoriasis.
Sprayable compositions comprising a combination of pharmaceutical actives and an oily phase
InactiveUS20050281749A1Good acceptability and toleranceChemically stableBiocideAerosol deliveryDermatological disordersOil phase
Sprayable, anhydrous and physically / chemically stable dermatological / pharmaceutical compositions, well suited for the treatment of a variety of dermatological disorders, notably psoriasis, contain: a) a therapeutically effective amount of a solubilized corticoid, notably dissolved clobetasol propionate; b) a therapeutically effective amount of a solubilized vitamin D derivative, notably dissolved calcitriol; and c) an oily phase which comprises one or more oils; formulated into d), a sprayable and topically applicable, dermatologically / pharmaceutically acceptable vehicle therefor.
Owner:GALDERMA SA
Sprayable pharmaceutical compositions comprising a corticoid and an oily phase
Anhydrous sprayable pharmaceutical / dermatological compositions containing, as active pharmaceutical agent, a corticoid, preferably clobetasol propionate, and an oily phase, are formulated in a physiologically acceptable medium, and are useful for the treatment of a variety of conditions and afflictions, notably psoriasis.
Owner:GALDERMA SA
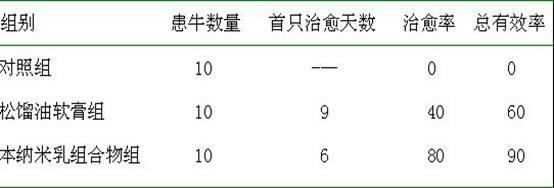
Nano-emulsion medicine for treating foot rot and preparation method thereof
InactiveCN102552339AHigh thermodynamic stabilityEasy to operateAntibacterial agentsHydroxy compound active ingredientsPropanoic acidCutin
The invention discloses a nano-emulsion medicine for treating foot rot. The grain diameter of the nano-emulsion is in a range of 1-100 nanometers and the nano-emulsion is composed of the following raw materials: 0.1-10.0% of pine tar, 0.1-6.0% of cinnamyl aldehyde, 0.1-6.0% of carvacrol, 0.1-5.0% of camphor oil, 0.01-0.1% of clobetasol propionate, 18.0-35.0% of surfactant, 0-7.0% of co-surfactant and residual amount of distilled water; and the sum of the mass percentages of the components is 100%. The nano-emulsion disclosed by the invention has the functions of dissolving cutin, relieving itching, diminishing inflammation, restraining, partially disinfecting and resisting corrosion, accelerating absorption and the like, and is mainly used for treating animal foot rot. According to the nano-emulsion medicine for treating the foot rot, the solubility of the medicine is increased, the medicinal stability and the biological utilization rate are improved, the dosage of the medicine is reduced, the skin transmission rate is higher than that of common preparations including a medicinal extract and the like, and the cost is low, so that the nano-emulsion medicine has a wide market prospect in a veterinary medicine field.
Owner:NORTHWEST A & F UNIV
Sprayable compositions comprising a combination of pharmaceutical active ingredients, an alcohol phase and an oily phase
InactiveUS20050281754A1Easy to useHigh acceptabilityCosmetic preparationsBiocideAlcoholAdditive ingredient
Sprayable, anhydrous and physically / chemically stable dermatological / pharmaceutical compositions, well suited for the treatment of a variety of dermatological disorders, notably psoriasis, contain: a) a therapeutically effective amount of a solubilized corticoid, notably dissolved clobetasol propionate; b) a therapeutically effective amount of a solubilized vitamin D derivative, notably dissolved calcitriol; and c) an alcohol phase; and d) an oily phase which comprises one or more oils; formulated into e), a sprayable and topically applicable, dermatologically / pharmaceutically acceptable vehicle therefor.
Owner:GALDERMA SA
Topically applicable anti-inflammatory O/W emulsions comprising pro-penetrating glycols
Stable, topically applicable, non-greasy and non-tacky oil-in-water (O / W) anti-inflammatory emulsions, useful, e.g., for the treatment of psoriasis, contain: a. a therapeutically effective amount of at least one steroidal anti-inflammatory agent, notably clobetasol propionate; b. a pro-penetrating system which includes at least one pro-penetrating glycol and at least one other pro-penetrating agent; and c. at least one polymeric emulsifier not sensitive to electrolytes, for example not an acrylate / C10-C30 alkyl acrylate copolymer, formulated into a topically applicable, pharmaceutically acceptable vehicle therefor.
Owner:GALDERMA SA
Use of a clobetasol spray formulation to treat psoriasis
InactiveUS20060239929A1Increased clinical benefitBeneficial therapeutic resultHydroxy compound active ingredientsAerosol deliveryAlcoholSURFACTANT BLEND
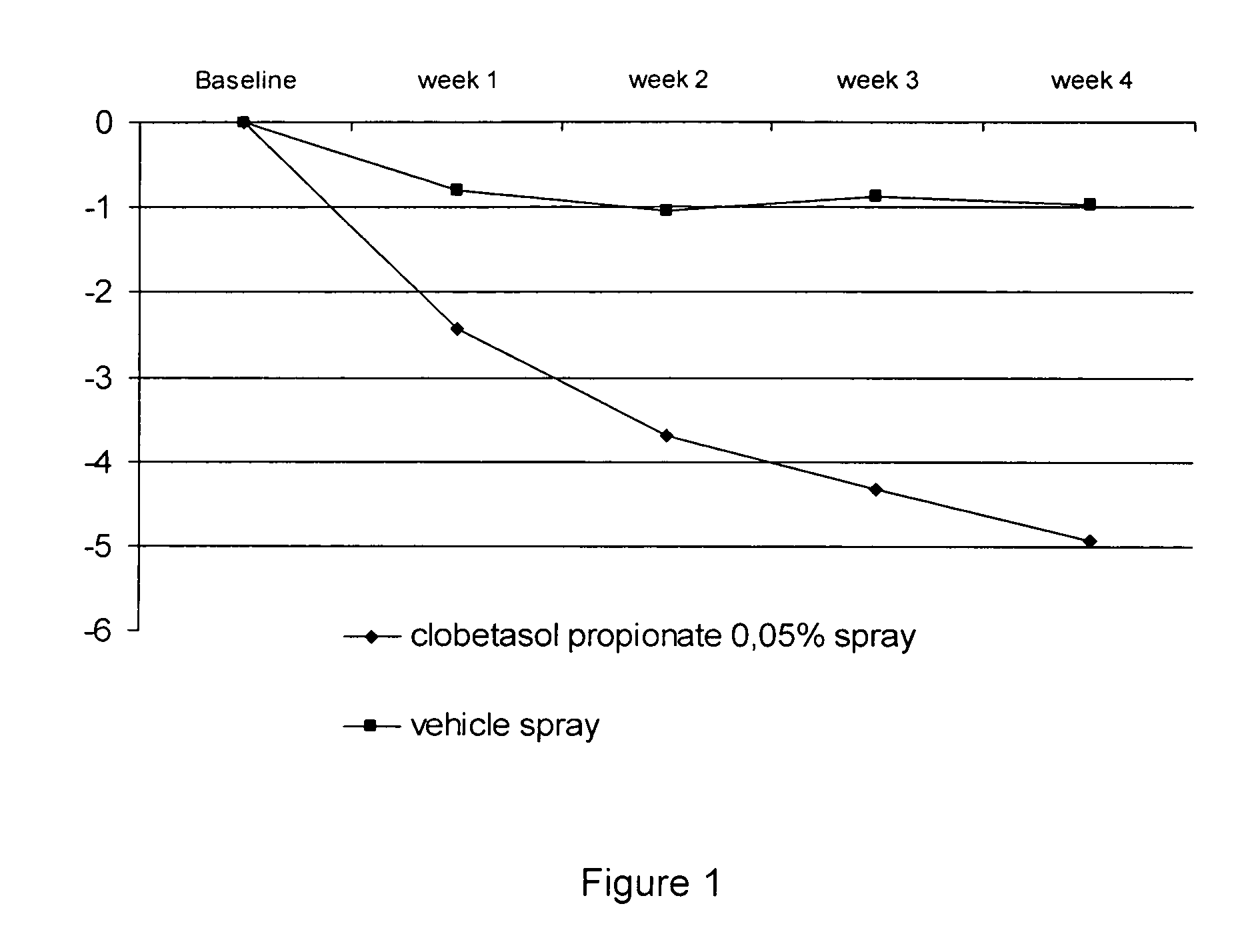
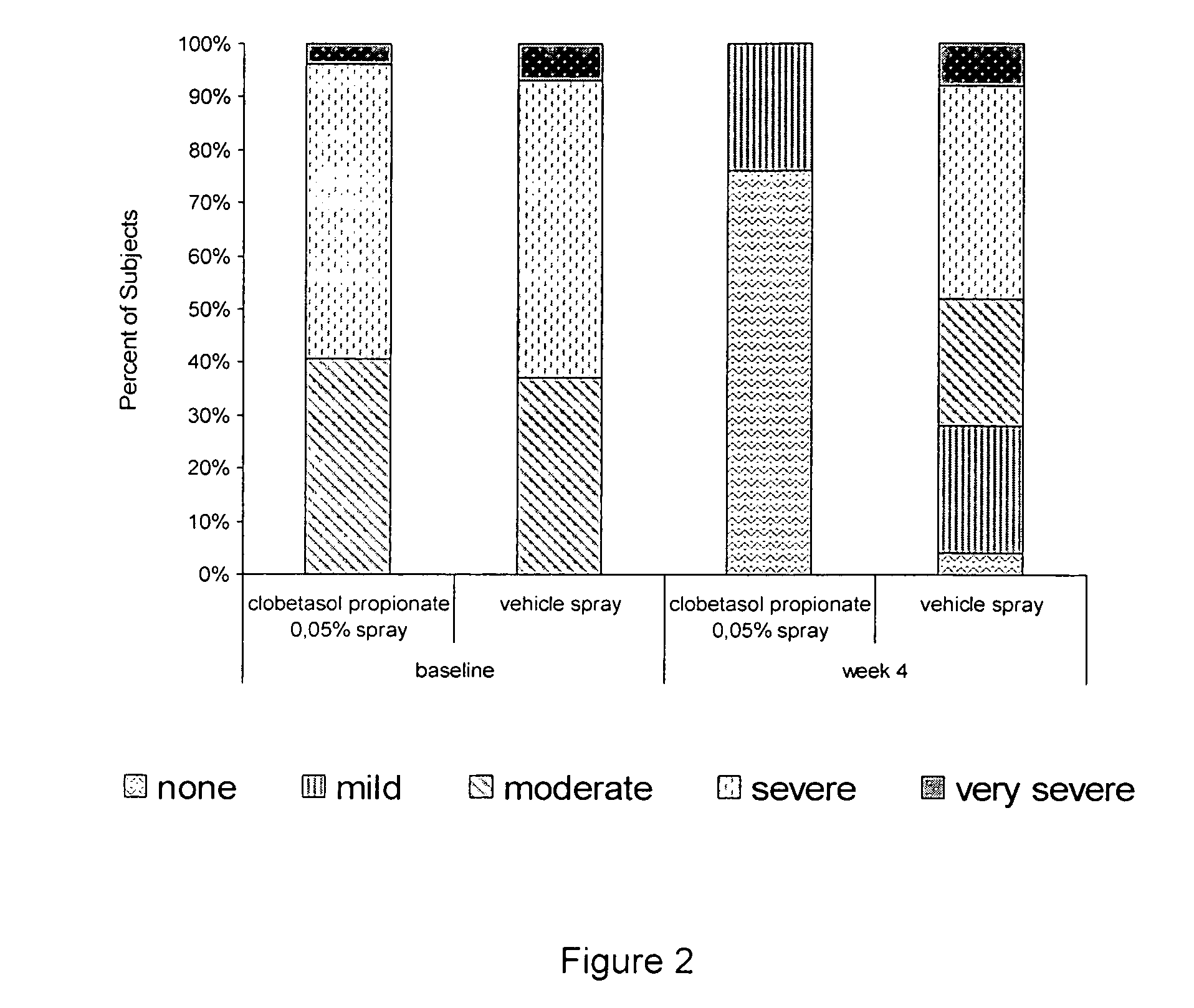
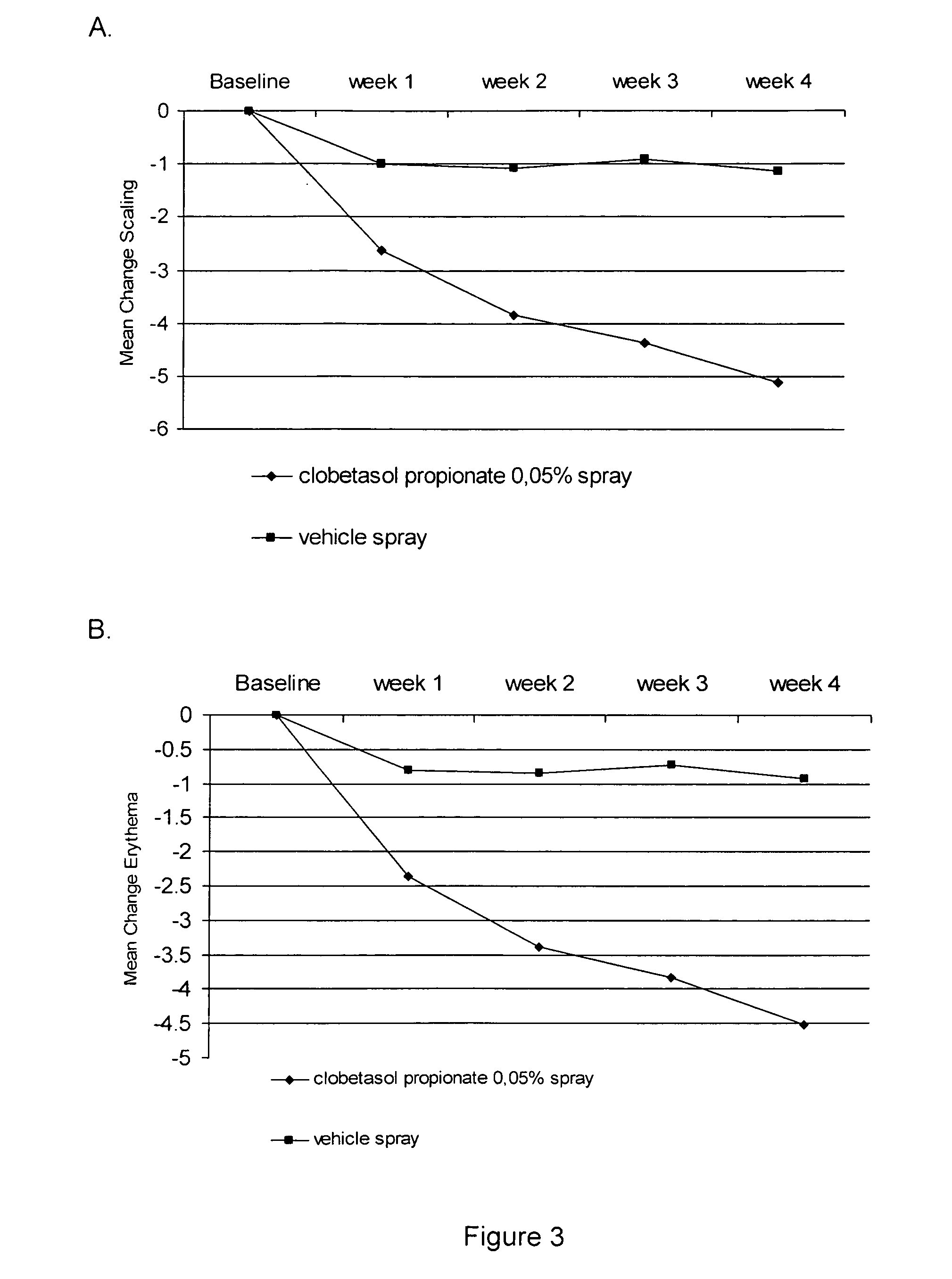
The present invention provides a method for treating psoriasis, by spraying onto the skin with psoriasis daily for at least 4 weeks a pharmaceutical composition containing an effective amount of clobetasol propionate. A preferred pharmaceutical composition containing clobetasol propionate, ethyl alcohol, isopropyl myristate, and anionic surfactant.
Owner:DOW PHARMA SCI INC +1
Calcitriol/clobetasol propionate compositions for the treatment of psoriasis
InactiveUS20050282792A1Good synergyOrganic active ingredientsDermatological disorderRegimenCalcitriol
Topically applicable pharmaceutical compositions useful for the treatment of psoriasis contain respective amounts of calcitriol and clobetasol propionate permitting a once-per-day effective regimen of topical application onto the part or parts of the skin affected by psoriasis.
Owner:GALDERMA SA
Topical formulation of low level clobetasol propionate for treating disorders of the skin and mucous membranes
InactiveUS20100249060A1Good chemical stabilityLong durabilityBiocideOrganic active ingredientsPolyethylene glycolGlycerol
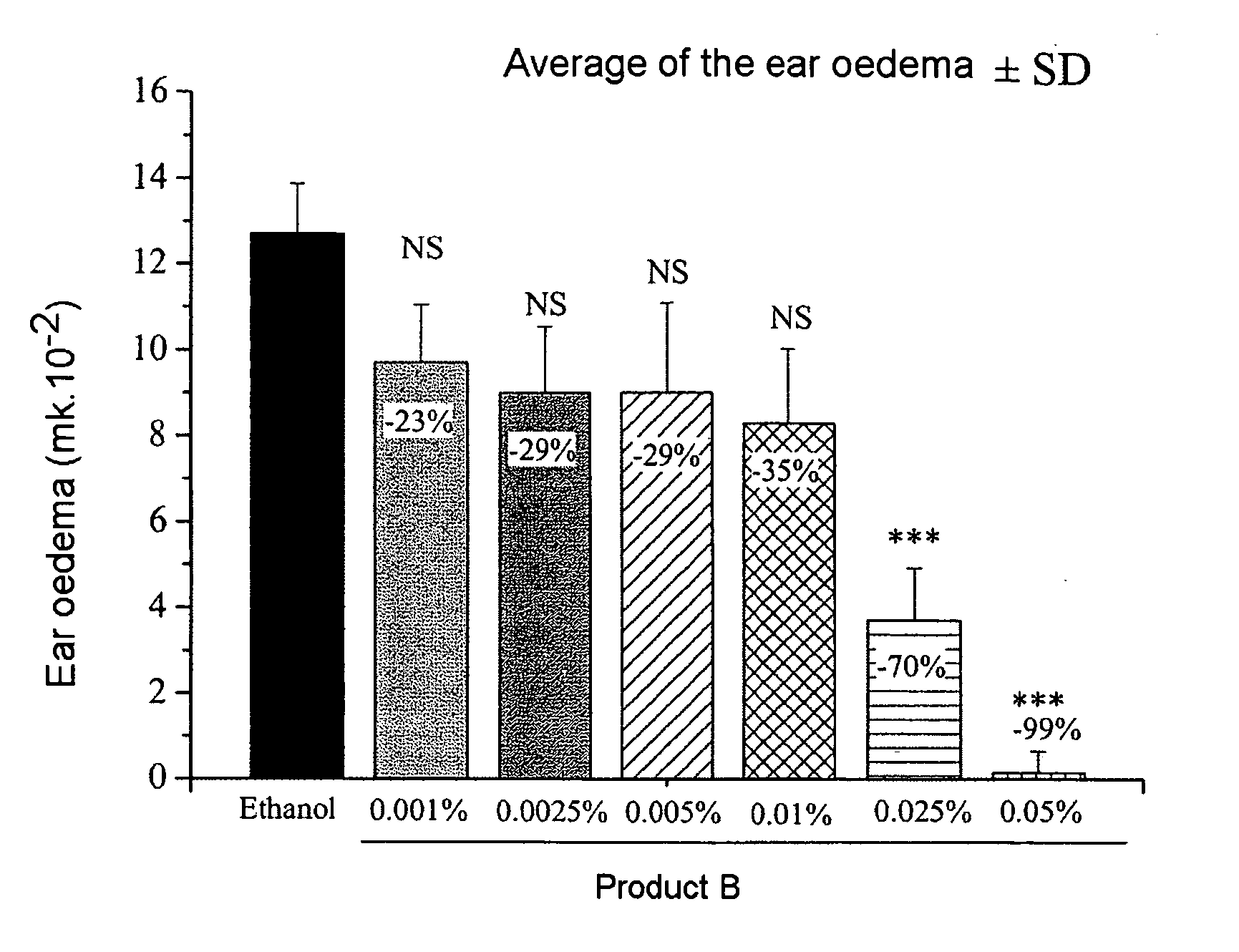
A new topical formulation is provided, with a high chemical stability, of for example a low dose clobetasol propionate, suitable for the topical treatment of skin and mucous membrane conditions associated with disorders including psoriasis, eczema, and other forms of dermatitis and also topical use associated with the mouth, such as lichen planus. The formulation includes an aqueous vehicle of based on propylene glycol as a solvent and moisture-retaining agent, and macrogol-glycerol hydroxystearate as a non-ionic emulsifier, being capable of holding surprisingly low concentrations of clobetasol. The vehicle holds concentrations about 0.005% to about 0.05% by weight of 17-clobetasol propionate, more preferably about 0.02 to 0.025%, even more preferably 0.025% by weight of 17-clobetasol propionate. The formulation has a good chemical stability, resulting in a long durability.
Owner:SMITH JAN G
Compound clobetasol propionate liposome and preparation thereof
ActiveCN102429913AEasy to storeIncrease resistanceHydroxy compound active ingredientsEmulsion deliveryLipid formationDisease
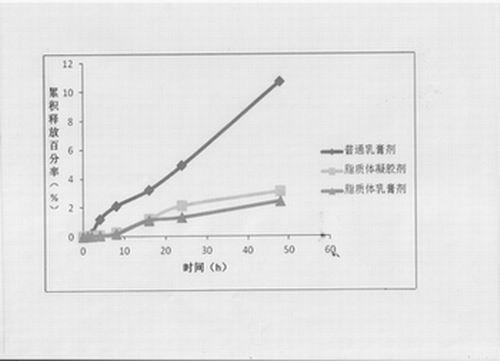
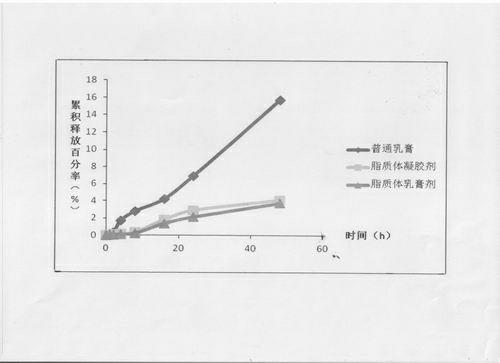
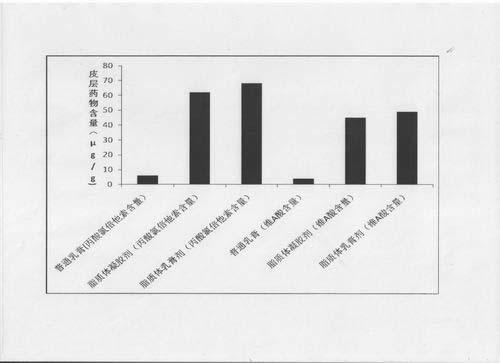
The invention provides a compound clobetasol propionate liposome and a preparation thereof, which are mainly used for treating diseases such as psoriasis vulgaris, dermatitis, eczema and the like. The liposome prepared from neutral synthetic phospholipids, lipids with positive charges, and cholesterol can coat clobetasol propionate and vitamin A acid simultaneously, and is added with a cream substrate or gel substrate to be prepared into compound clobetasol propionate liposome cream or gel. Compared with the common cream, the liposome preparation has the advantages that: the amount of the medicine retained in skin is larger, the skin penetration rate is lower, the content of the medicine in local skin can be improved, the treatment index is improved, and transdermal absorption dose is reduced, so that the toxic and side effects of the medicine are reduced.
Owner:JIANGSU SEMPOLL PHARMA
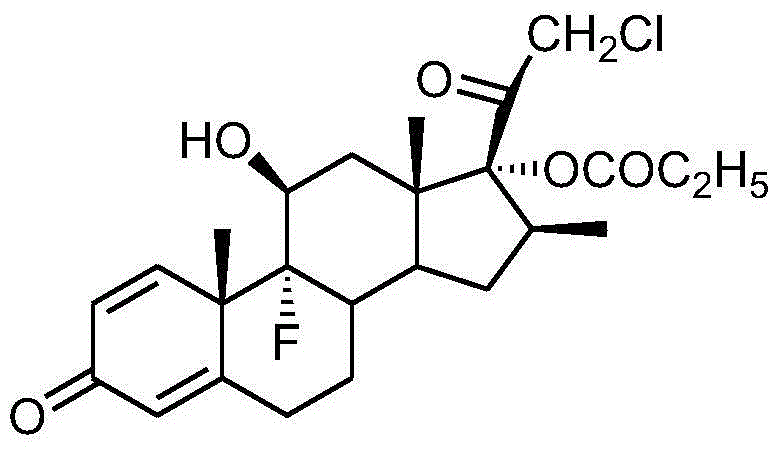
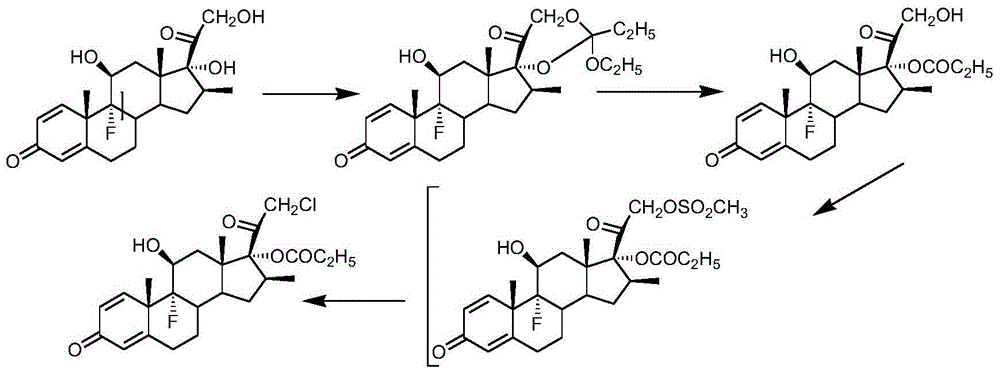
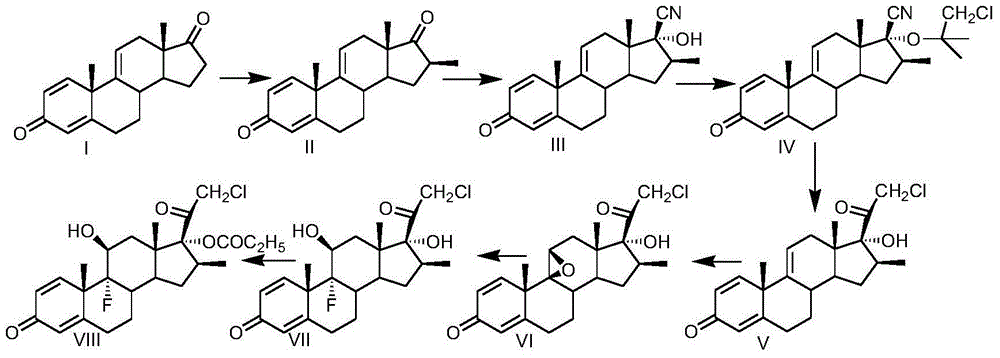
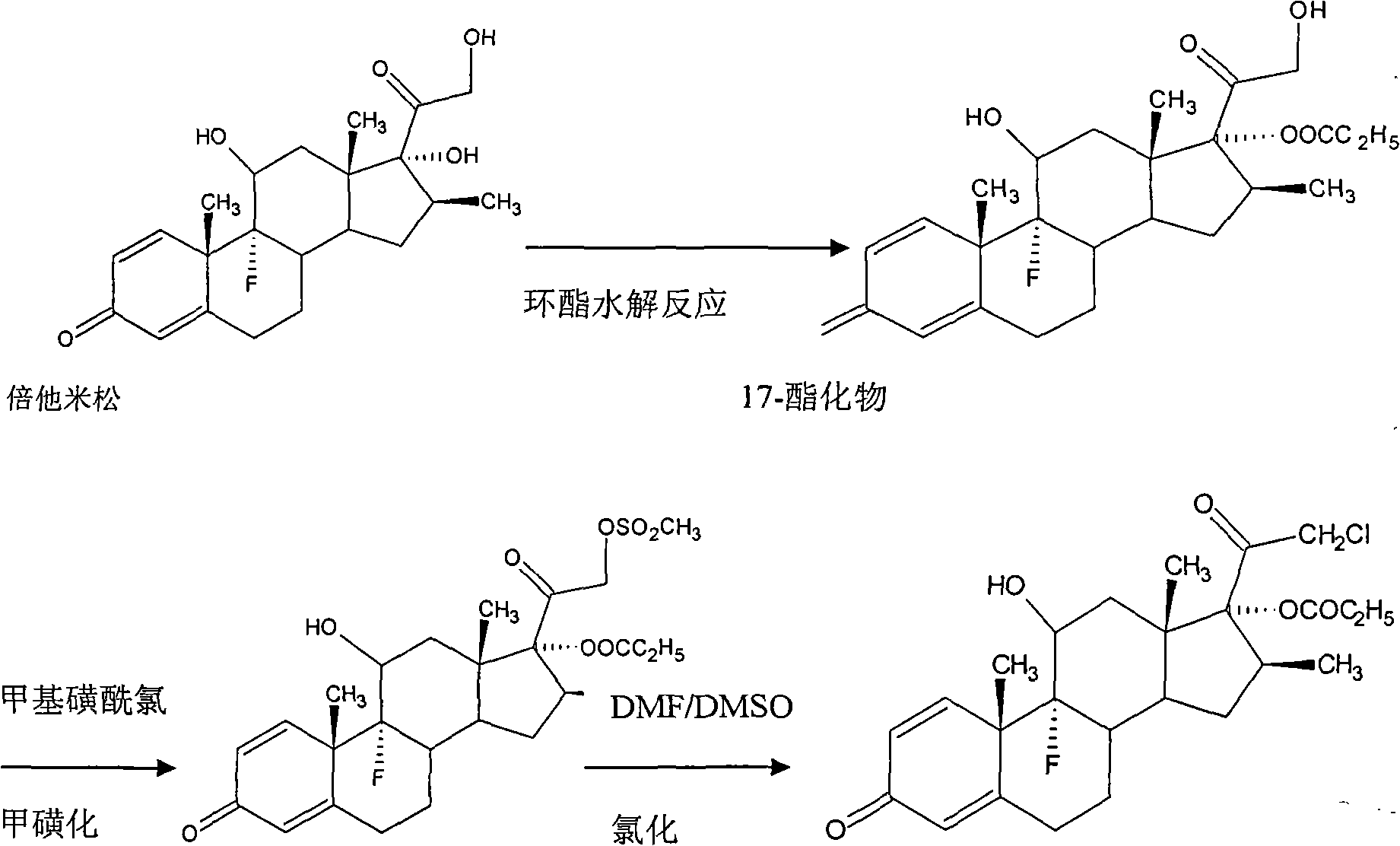
Preparation method of clobetasol and preparation method of clobetasol propionate
The invention discloses a preparation method of clobetasol and the preparation method of clobetasol propionate. The preparation method of the clobetasol comprises the following steps: by taking a compound I, namely 1,4,9(11)-triene androstane-3,17-diketone as an initial raw material, performing a methylation reaction, a cyan substitution reaction, a siloxy protection reaction, an intramolecular nucleophilic substitution reaction, a bromoepoxy reaction and a fluorination reaction to prepare a compound VII which is clobetasol. The compound VII is subjected to a propyl esterification reaction to prepare a compound VIII which is clobetasol propionate. According to the preparation method disclosed by the invention, since relatively basic initial raw materials which are cheap are used, each step of reaction is relatively easy to implement and high yield is achieved; the operation of multi-step protection and deprotection is simplified; moreover, 21 sites of fluorine are directly arranged in one step during arrangement of a side chain, and multiple steps of reaction for arranging the 21 sites of fluorine in the prior art are directly avoided, so that the synthetic route is greatly shortened, the total yield is increased, the product quality is improved and the production cost is greatly lowered.
Owner:江西赣亮医药原料有限公司
Methods of Treating Diseased Tissue
InactiveUS20120109042A1Good effectElectrotherapyPharmaceutical delivery mechanismGeneralized psoriasisContinuous use
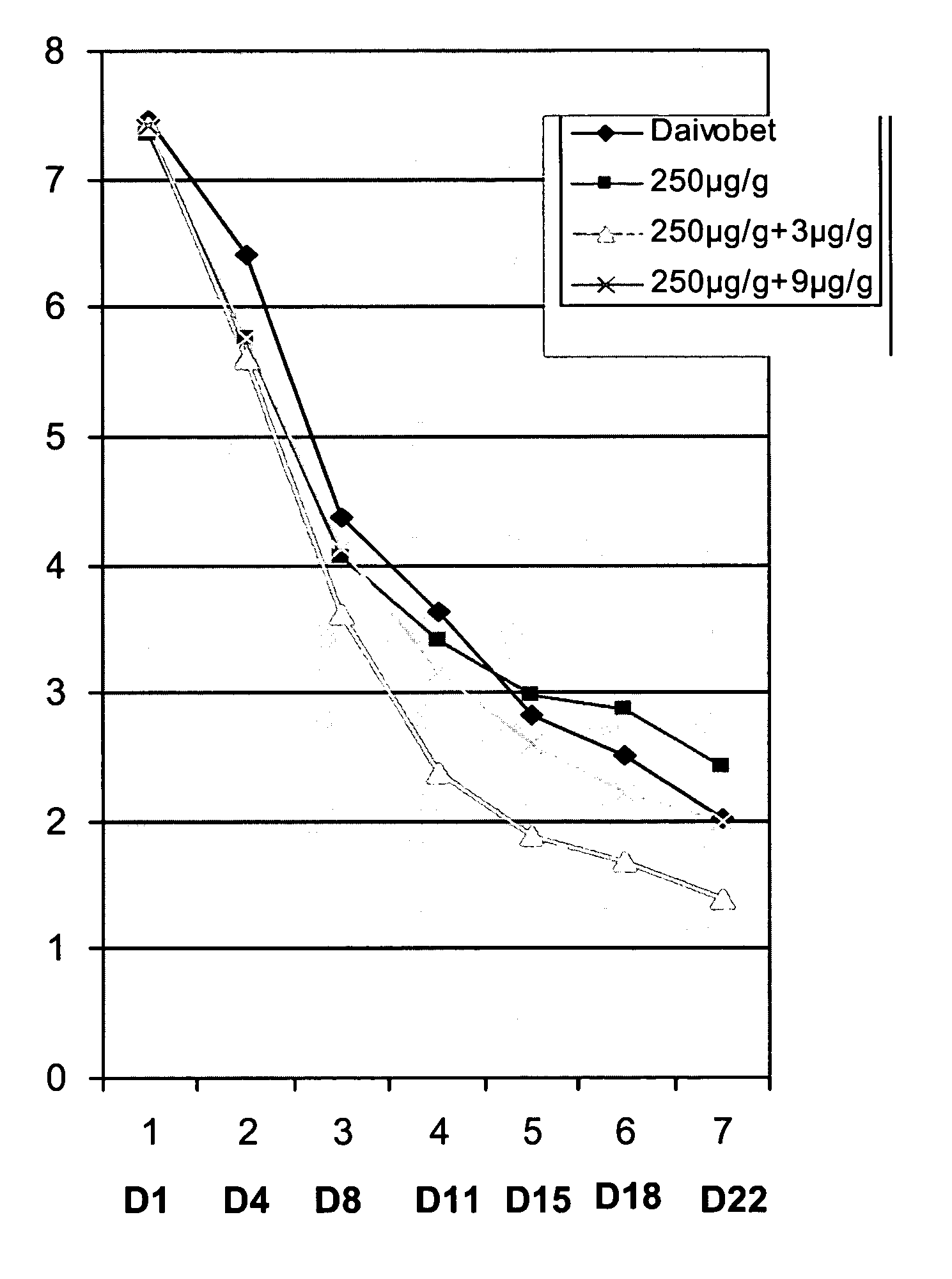
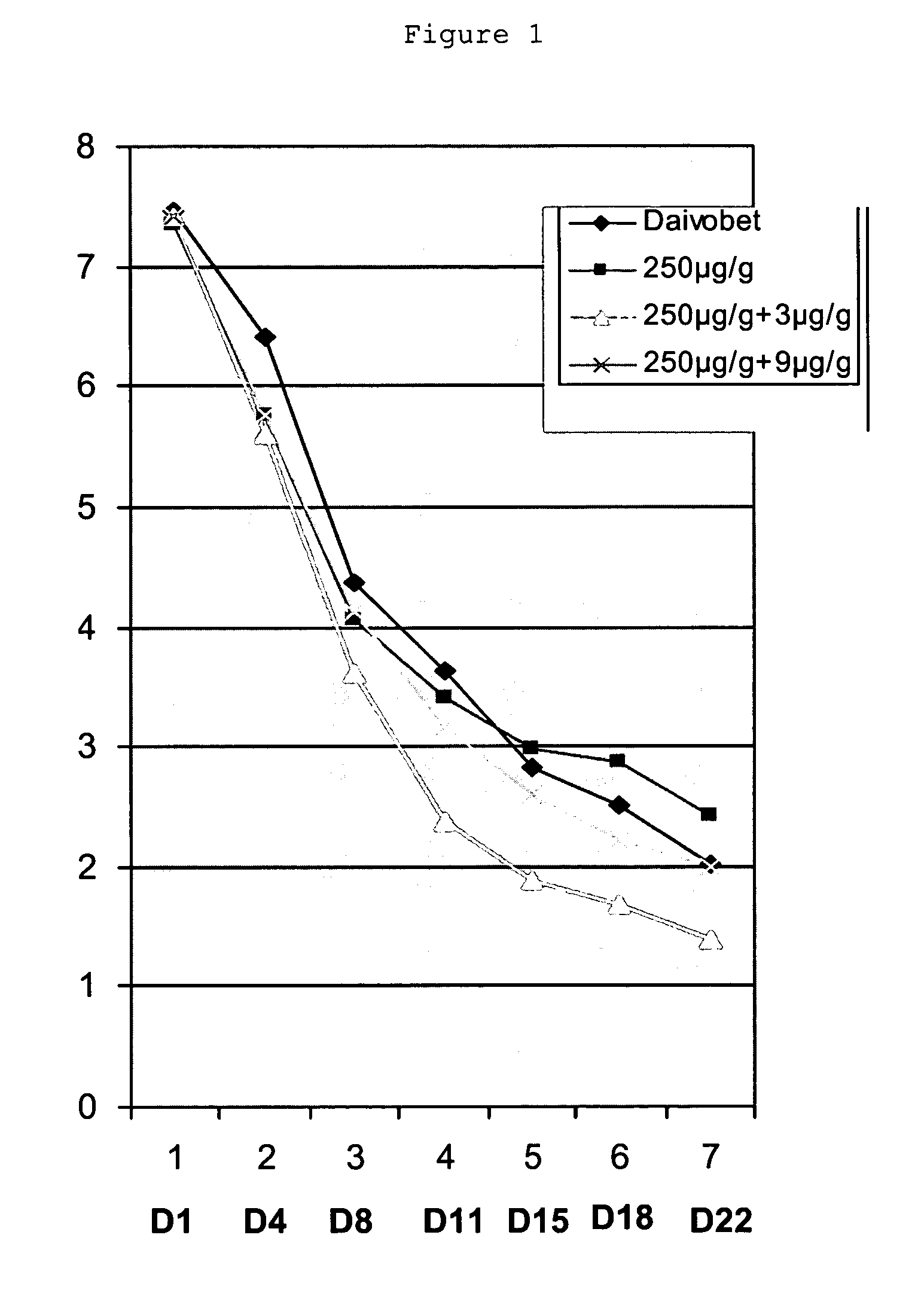
Two or three external therapeutic options such as clobetasol propionate spray, calcitriol ointment, and excimer laser phototherapy are used to treat generalized psoriasis with systemic safety and better efficacy than any systemic or biologic agent. Combinations of these agents, for example used in parallel and / or series for specific durations, can achieve a significant result: a PASI 75 response after only four weeks and / or a PASI 95-100 response after only six weeks.
Owner:RGT UNIV OF CALIFORNIA
Preparation method of intermediate of steroidal drug with 16-beta-methyl
InactiveCN101851264AEasy accessSimple processSteroidsBetamethasone Sodium PhosphateBetamethasone valerate
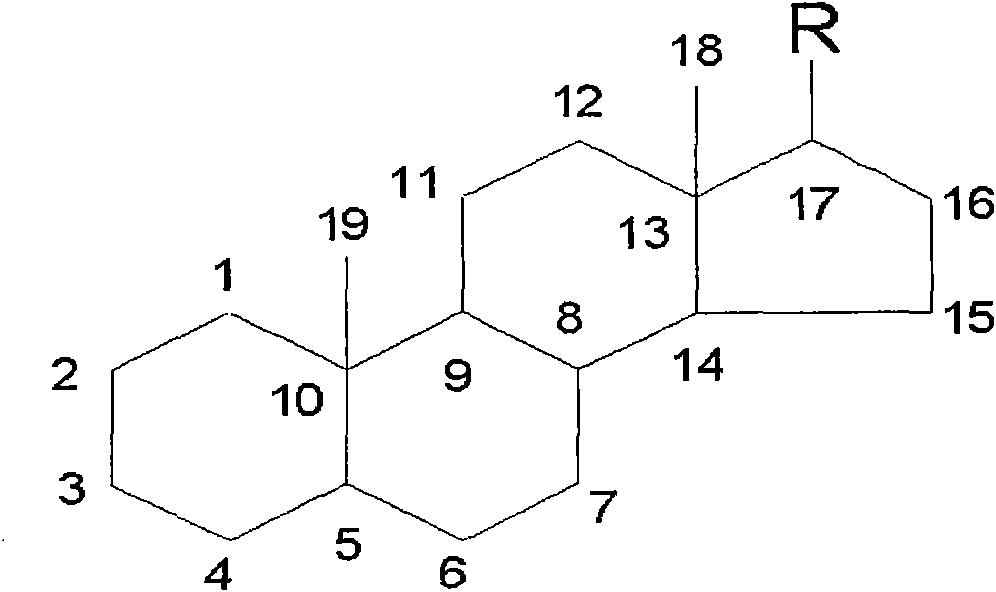
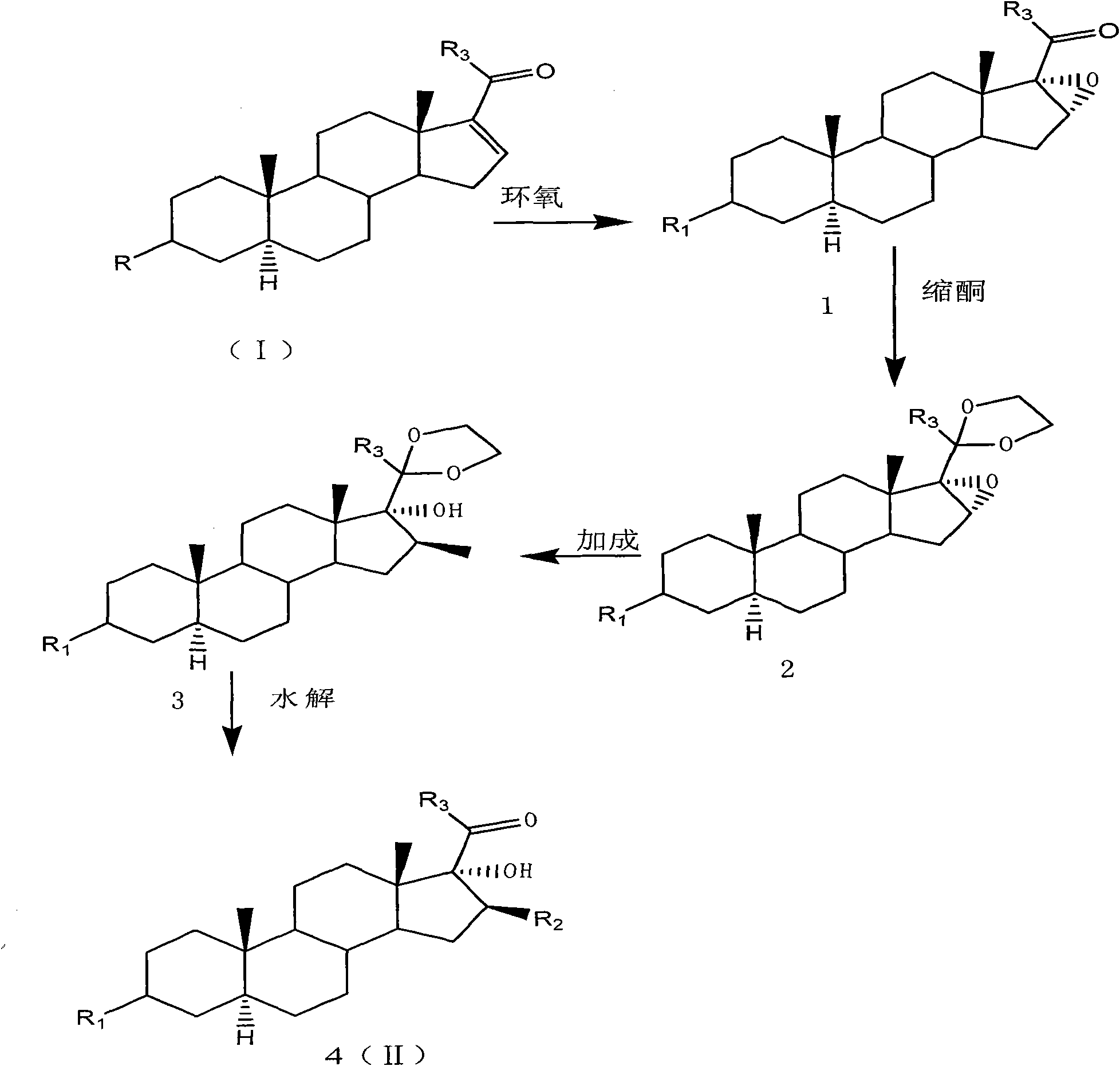
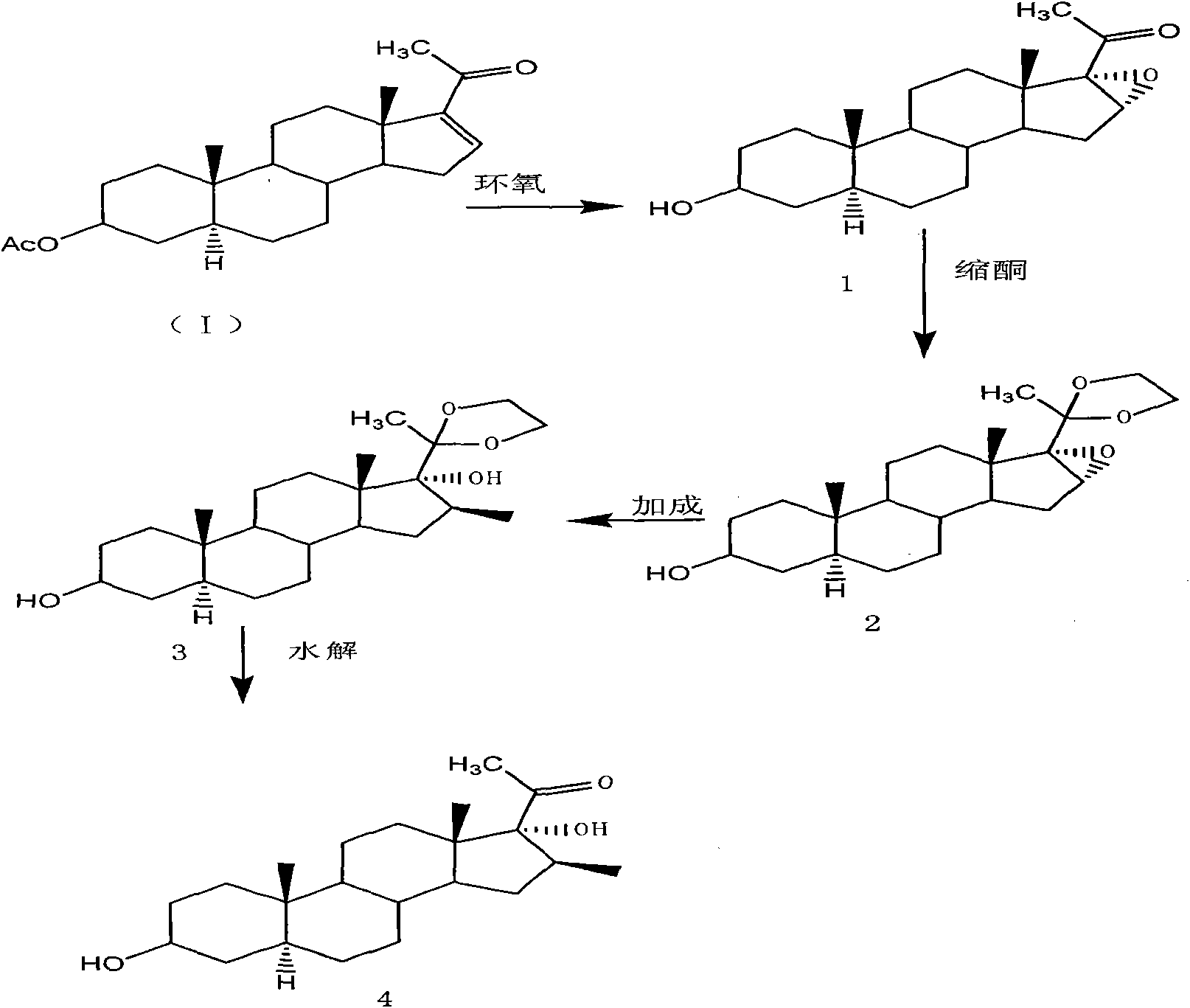
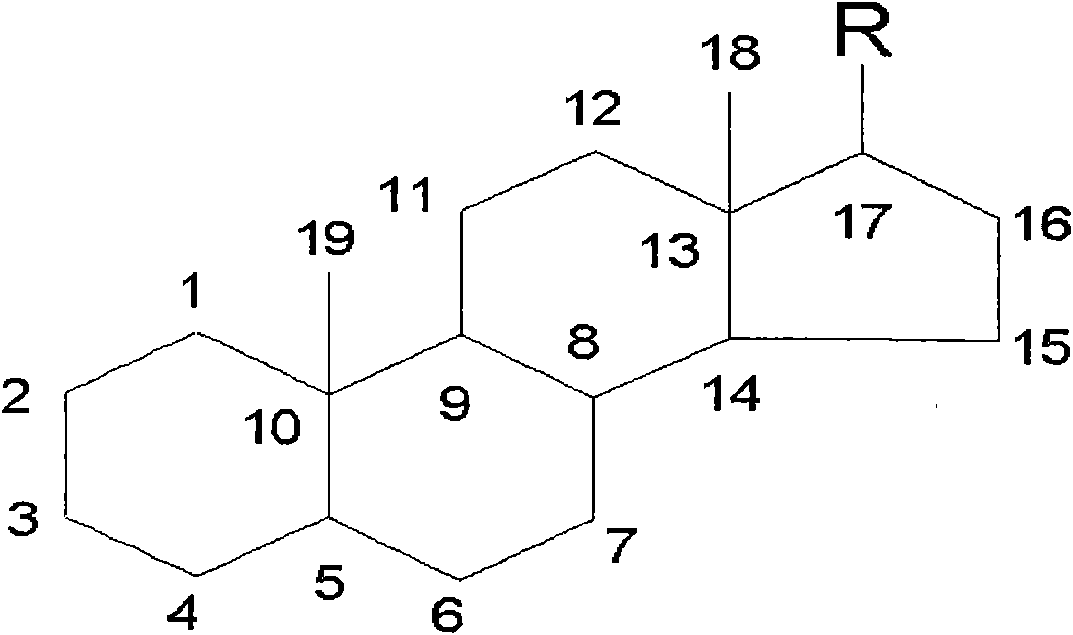
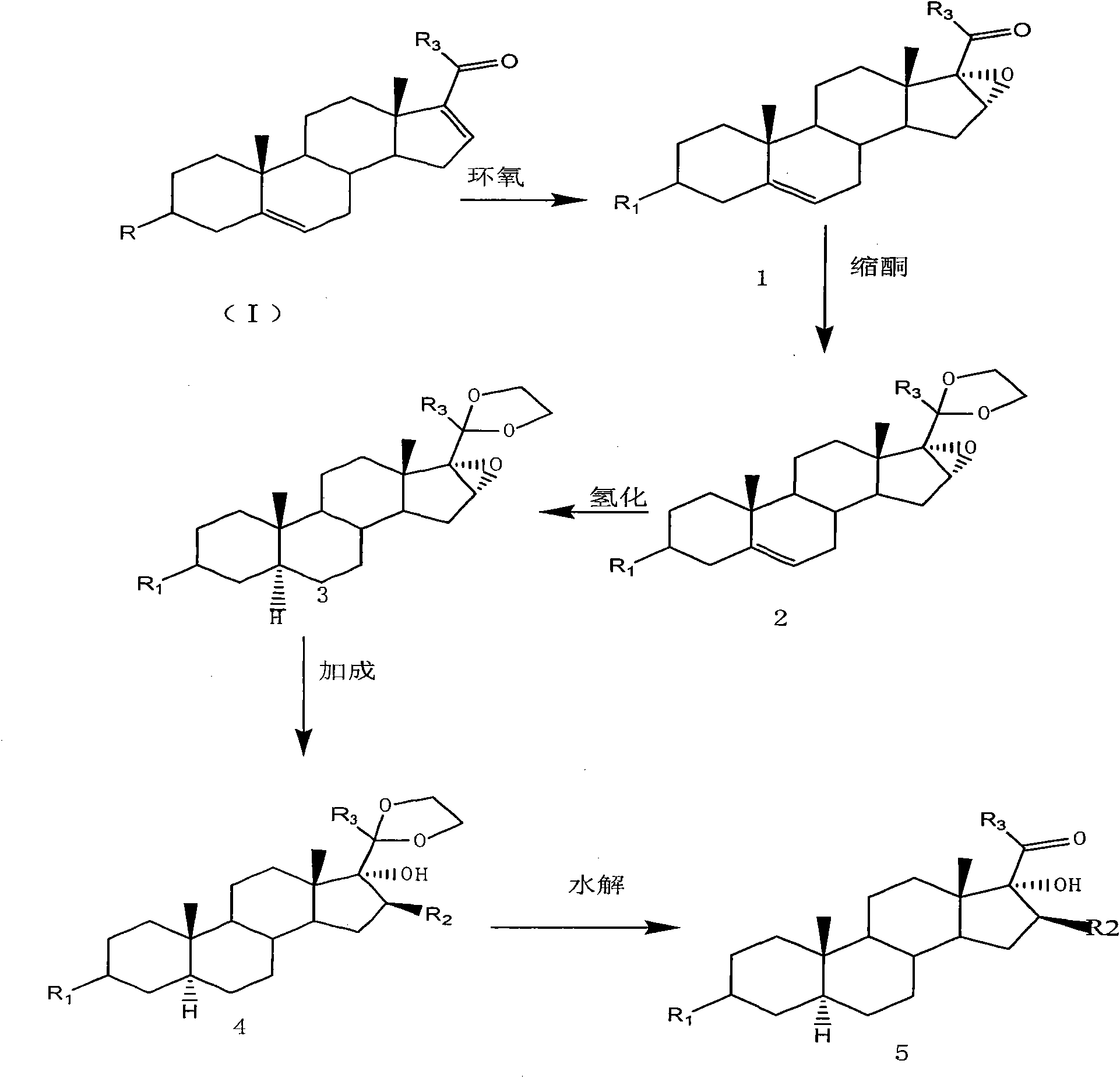
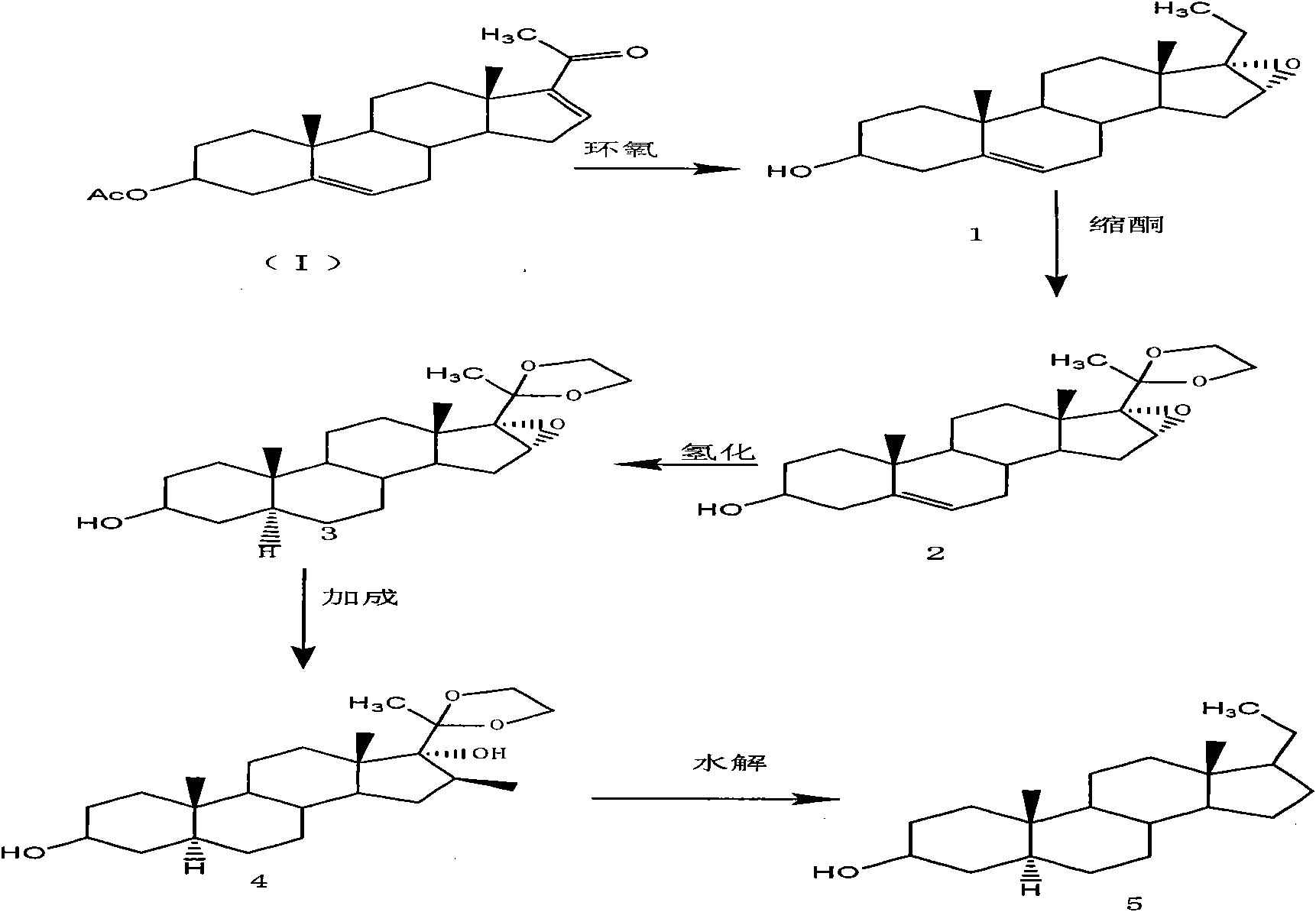
The invention provides a preparation method of an intermediate of a steroidal drug with a 16-beta-methyl. In the method, a steroidal compound I is used as a starting material, and a steroidal compound II of the intermediate of the steroidal drug with the 16-beta-methyl can be prepared through epoxy reaction, ketal reaction, addition reaction and hydrolysis reaction for transformation of Position 3, Position 16, Position 17 and Position 20 or Position 16, Position 17 and Position 20. The steroidal compound II with the 16-beta-methyl is generally applied in preparation of the glucocorticoid drugs commonly used in clinical operation, such as betamethasone, betamethasone sodium phosphate, betamethasone acetate, clobetasol propionate, beclometasone dipropionate, betamethasone valerate and the like. In the formulas of the steroidal compound I and the steroidal compound II, R represents OH or OCOCH3, R1 represents OH, R2 represents CH3, and R3 represents CH3.
Owner:GUANGXI WANDE PHARMA
Compound clobetasol propionate mixed micellar solution and preparation method thereof
ActiveCN102641276ASimple preparation processIncrease temperatureHydroxy compound active ingredientsPharmaceutical delivery mechanismDiseaseMixed micelle
The invention provides a compound clobetasol propionate mixed micellar solution. Phosphatide and cholate are mainly utilized to prepare the mixed micellar solution which is used for packaging clobetasol propionate and tretinoin. The mixed micelle solution can be simply prepared, and the medicament stability can be guaranteed in the process of preparation. By preparing a mixed micelle system from the phosphatide and the cholate, the problem of medicament dissolution is solved. Compared with a common ointment, the mixed micelle solution has a higher retention volume on skins, and the quantity of the medicament on local skins and the therapeutic index can be increased. The mixed micelle solution can be further prepared into an ointment and a gel, and is mainly used for treating psoriasis vulgaris, dermatitis, eczema, etc.
Owner:JIANGSU SEMPOLL PHARMA
Preparation method of intermediate of steroidal drug with 16-beta-methyl
InactiveCN101851263AEasy accessSimple processSteroidsBetamethasone Sodium PhosphateHydrogenation reaction
The invention provides a preparation method of an intermediate of a steroidal drug with a 16-beta-methyl. In the method, a steroidal compound I is used as a starting material, and a steroidal compound II of the intermediate of the steroidal drug with the 16-beta-methyl can be prepared through epoxy reaction, ketal reaction, hydrogenation reaction, addition reaction and hydrolysis reaction for transformation of Position 3, Position 5, Position 6, Position 16, Position 17 and Position 20 or Position 5, Position 6, Position 16, Position 17 and Position 20. The steroidal compound II with the 16-beta-methyl is generally applied in preparation of the glucocorticoid drugs commonly used in clinical operation, such as betamethasone, betamethasone sodium phosphate, betamethasone acetate, clobetasol propionate, beclometasone dipropionate, betamethasone valerate and the like. In the formulas of the steroidal compound I and the steroidal compound II, R represents OH or OCOCH3, R1 represents OH, R2 represents CH3, and R3 represents CH3.
Owner:GUANGXI WANDE PHARMA
Compound preparation fortreating psoriasis vulgaris and its preparation process
InactiveCN1234365CDoes not affect biological activityReduce irritation responseDermatological disorderAnhydride/acid/halide active ingredientsClinical efficacyTretinoin
A medicine for treating psoriasis vulgaris contains clobetasol propionate and vitamin A acid. Its preparing process is also disclosed. Its advantages are high curativ effect, and low toxic by-effect.
Owner:JIANGSU SEMPOLL PHARMA
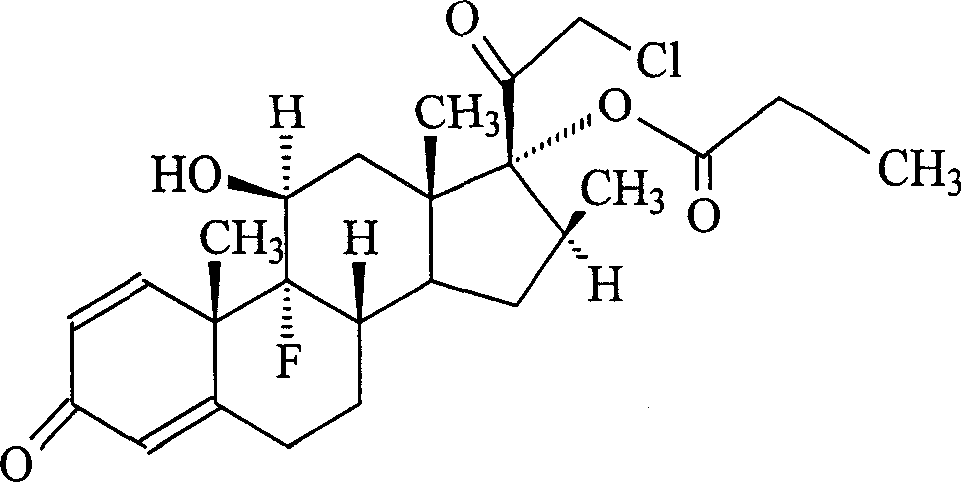
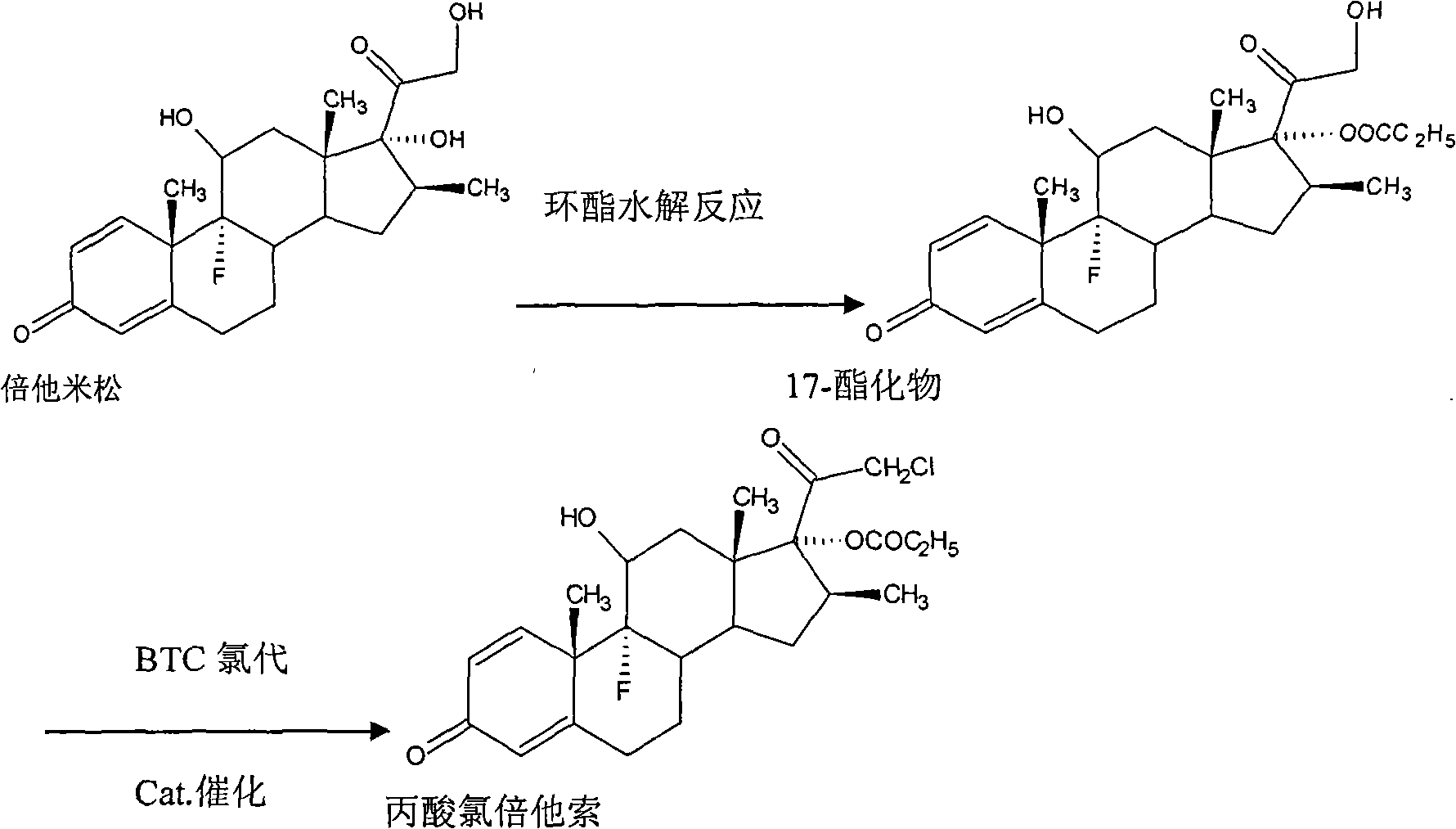
Preparation method of propionic acid clobetasol
The invention discloses a preparing method of chlorotestosterone betaprodine, which comprises the following steps: blending betamisong 17-propionic ester sulphonation and anhydrous lithium chloride with the rate at 1: 1-2; dissolving in the DMF to do chloridization reaction; elutriating through icy water; drying through centrifuging to obtain rough product; dissolving rough product in the carbinol or alcohol; adding activated charcoal; decoloring; filtering; recycling activated charcoal; condensing the filtrate; crystallizing; dehydrating; drying to obtain the product.
Owner:ZHEJIANG DINGTAI PHARMA
Antimycotic externally used drug
InactiveCN101129409APromote regenerationGood curative effectOrganic active ingredientsAntimycoticsDiseaseBeriberi
The invention discloses an externally-used medicament for treating skin mycosis diseases, which is prepared from the raw materials of (by weight percent) terbinafine hydrochloride or miconazole or ketoconazole 0. 5-2%, Clobetasol Propionate 0. 01-0. 2%, neomycin sulphate 0. 3-1%, bee glue 5-25%, and balancing distilled water.
Owner:程贵昌
Compound preparation fortreating psoriasis vulgaris and its preparation process
InactiveCN1517092ADoes not affect activityDoes not affect anti-inflammatory effectOrganic active ingredientsDermatological disorderTretinoinCurative effect
A medicine for treating psoriasis vulgaris contains clobetasol propionate and vitamin A acid. Its preparing process is also disclosed. Its advantages are high curativ effect, and low toxic by-effect.
Owner:JIANGSU SEMPOLL PHARMA
Liquor preparations for removing dandruff and method for preparing the same
InactiveCN101095799APrevent regenerationRegulate secretionHydroxy compound active ingredientsDermatological disorderKetoconazoleEthanol
The invention discloses a solution for removing dandruff and its preparing process, wherein the solution is prepared from rhubarb horsetails, flavescent sophora root, Chinese dittany bark, poria cocos, rough gentian, boneol, menthol, ketoconazole, Clobetasol Propionate, propylene glycol, glycerin, azone, polysorbate -80 and sodium benzoate through charging the first five Chinese herbs into water, boiling, concentrating the filtrate, charging ketoconazole, Clobetasol Propionate, boneol, menthol and auxiliary materials, finally keeping constant volume by charging ethanol.
Owner:SHANDONG XUANZHU PHARMA TECH CO LTD
Transdermal drug delivery system and method of using the same
InactiveUS8900626B2Effective treatmentImprove permeabilityOrganic active ingredientsBiocideDiseaseActive agent
A transdermal drug delivery system comprising a steroid as an active agent, wherein the steroid may be clobetasol propionate, betamethasone dipropionate, amcinonide, or loteprednol etabonate. The transdermal drug delivery system also comprises a pressure-sensitive adhesive layer and a support, wherein the steroid is present in the pressure-sensitive adhesive layer, and wherein the pressure-sensitive adhesive layer is provided on a support. The transdermal drug delivery system may be applied onto the eyelid of a patient in need thereof, in order to treat a disease of the eyelid, such as chalazion, blepharitis or meibomian gland dysfunction.
Owner:SENJU USA
A kind of oil-in-water type compound ketoconazole nano medicine and preparation method thereof
InactiveCN102283850ASpray evenlyApply evenlyAntibacterial agentsOrganic active ingredientsDiseasePropanoic acid
The invention discloses an oil-in-water compound ketoconazole nano-medicament. The grain diameter of the medicament is 1-100nm. The oil-in-water compound ketoconazole nano medicament comprises the following raw materials in percentage by weight: 0.01-8.0 percent of ketoconazole, 0.01-10 percent of eugenol, 0.001-5.0 percent of clobetasol propionate, 25.0-45.0 percent of surfactant, 0-10.0 percentof cosurfactant, 0.1-25.0 percent of oil and the balance of distilled water. The oil-in-water compound ketoconazole nano-medicament can be used for treating stubborn skin mycotic infection, mycodermatitis, chronic mucocutaneous candidiasis, dermatitis blastomycosis, coccidioidomycosis, histoplasmosis, chromoblastomycosis and paracoccidioidomycosis, and dermatomycosis and tinea versicolor caused by dermatophyte and microzyme as well as trichophyton disease, scytitis and pruritus. The oil-in-water compound ketoconazole nano-medicament has the advantages of strong osmosis, favorable stability, favorable infiltrating property, durable acting time, remarkable effect, low cost, simple preparation method and convenience in popularization and application.
Owner:NORTHWEST A & F UNIV
Method for synthesizing clobetasol propionate intermediate
ActiveCN101812107AReduce usageEmission reductionSteroidsReaction temperatureMethanesulfonyl chloride
The invention discloses a method for synthesizing clobetasol propionate, which belongs to the technical field of synthesis of steroid medicaments in the pharmaceutical chemistry and comprises the following steps: (1) dissolving betamethasone-17-ester into acetone solution; (2) adding a catalyst and BTC (bis(trichloromethyl) carbonate) for carrying out chloroacetic reaction at the reaction temperature of 30-50DEG C for 2-5 hours; and (3) after reaction, carrying out reduced pressure concentration, elutriation, filtering and drying. Compared with the traditional process, the yield can be improved by 6 percent, the cost is reduced by 20 percent and the quality is remarkably improved. The using amount of original auxiliary materials are reduced by 20 percent, the using amount of a toxic material of methanesulfonyl chloride can be reduced by 1 ton each year, the using amount of DMF (Dimethyl Formamide) can be reduced by 17 tons and the using amount of pyridine can be reduced by 6.3 tons, the economic cost can be totally saved by 366,000 yuan and waste water drain can be reduced by 310tons / year; and in addition, the invention greatly reduces the pressure for protecting the environment and can effectively reduce the hazard on human body and the pollution on the environment.
Owner:ZHEJIANG XIANJU XIANLE PHARMA
Externally applied medicament for treating vitiligo
InactiveCN101703620AImprove efficacyEasy to useOrganic active ingredientsPharmaceutical non-active ingredientsFiltrationAllergy
The invention provides an externally applied medicament for treating vitiligo, which relates to medicaments for treating vitiligo. The medicament consists of dark plum fruits, angelica dahurica roots, green husk, green walnut shells, lithospermum, Chinese arborvitae twig, malaytea scurfpea fruits, green eggplant, Chinese ilex leaves, Chinese dodder seeds, clobetasol propionate, laurocapram, chloroform and 90 percent alcohol. A making process comprises: a, soaking the dark plum fruits and the other 10 raw materials in alcohol for 5 to 7 days and forming Chinese medicinal liquid through filtration; b, dissolving clobetasol propionate with chloroform; c, adding clobetasol propionate solution and laurocapram to the Chinese medicinal liquid; and d, adding distilled water for dilution. In general, the raw medicinal materials cooperate to improve immunity by expanding blood vessels, releasing toxin and dispelling wind; brown pigment of the medicament is fully utilized and absorbed; tyrosinase is generated by stimulating skin so as to regenerate pigment; and the pigment is stabilized through allergy resistance. The medicament has the advantages of capability of treating both primary and secondary symptoms, rapid efficacy, safety, reliability, using convenience and good therapeutic effects. 110 clinical trials show that the total effective rate of the medicament reaches 94.5 percent.
Owner:秦剑
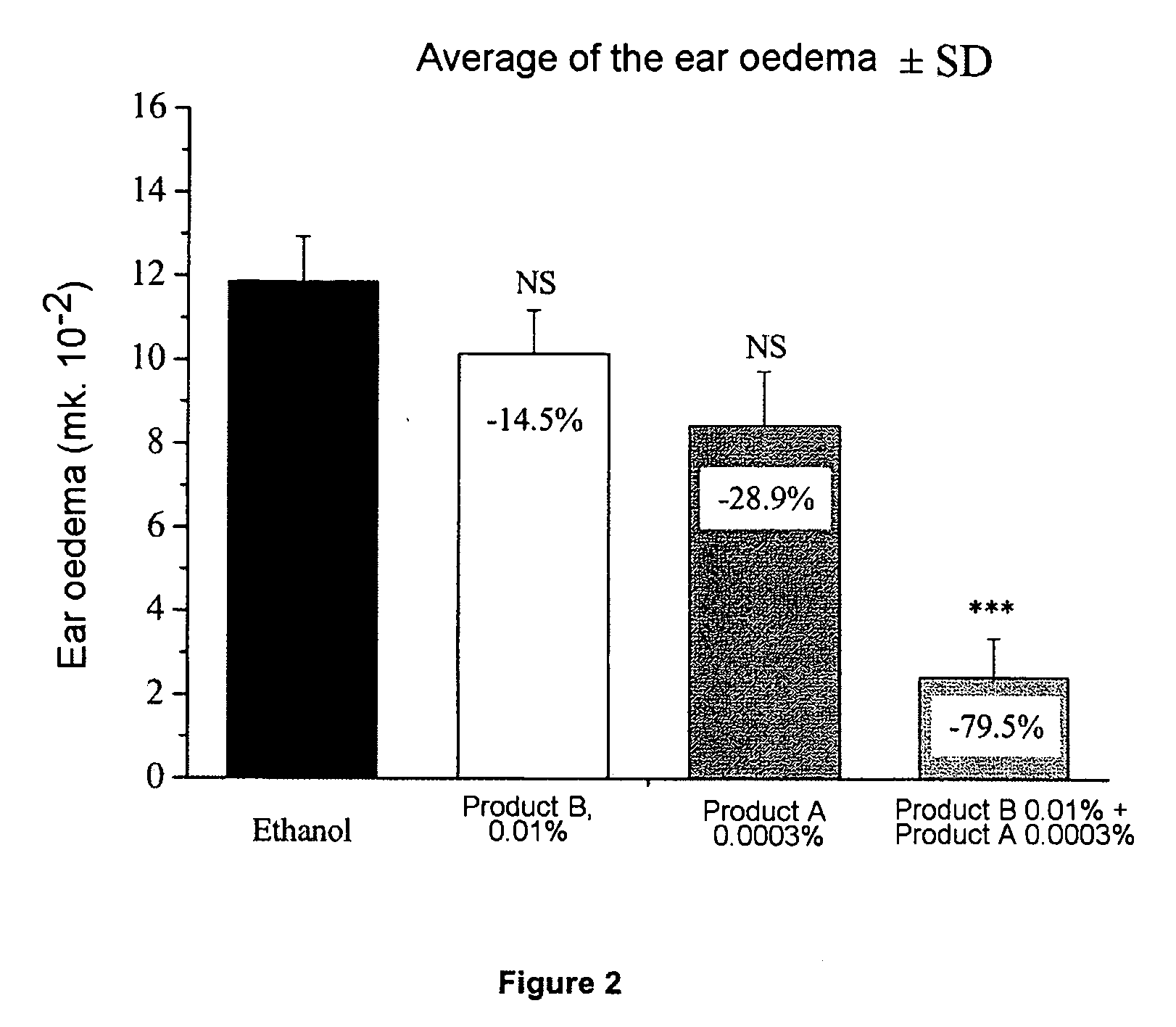
Pharmaceutical compositions comprising calcitriol and a clobetasol propionate
Topically applicable pharmaceutical gel, cream, lotion, solution or ointment compositions contain synergistically effective amounts of a) calcitriol and b) clobetasol propionate, formulated into a topically applicable, pharmaceutically acceptable medium therefor, and are useful for the treatment of such dermatological afflictions or conditions as psoriasis, atopic dermatitis, contact dermatitis and seborrhoeic dermatitis.
Owner:GALDERMA SA
Topical formulation of low level clobetasol propionate for treating disorders of the skin and mucous membranes
InactiveUS20120238535A1Good chemical stabilityLong durabilityOrganic active ingredientsAerosol deliveryPolyethylene glycolGlycerol
A new topical formulation is provided, with a high chemical stability, of for example a low dose clobetasol propionate, suitable for the topical treatment of skin and mucous membrane conditions associated with disorders including psoriasis, eczema, and other forms of dermatitis and also topical use associated with he mouth, such as lichen planus. The formulation includes an aqueous vehicle of based on propylene glycol as a solvent and moisture-retaining agent, and macrogol-glycerol hydroxystearate as a non-ionic emulsifier, being capable of holding surprisingly low concentrations of clobetasol. The vehicle holds concentrations about 0.005% to about 0.05% by weight of 17-clobetasol propionate, more preferably about 0.02 to 0.025%, even more preferably 0.025% by weight of 17-clobetasol propionate. The formulation has a good chemical stability, resulting in a long durability.
Owner:SMITH JAN G
Clobetasol spray
InactiveUS20080102038A1Reduce stimulationReduce solubilityBiocideOrganic active ingredientsPreservativeDimethyl isosorbide
A spray foaming dosage form comprising clobetasol propionate, dimethyl isosorbide, propylene glycol, polysorbate, sodium dodecyl sulphate, buffer, optional preservative, optional further excipients, and water.
Owner:NUPHARM LAB
Clobetasol Propionate Shampoos for the Treatment of Seborrheic Dermatitis of the Scalp
InactiveUS20080275014A1Treatment safetyOrganic active ingredientsCosmetic preparationsSeborrheic dermatitisScalp
Seborrheic dermatitis is effectively / safely treated by topically applying a corticosteroid shampoo, notably a clobetasol propionate shampoo, onto the scalp of a human subject afflicted therewith.
Owner:GALDERMA SA
Externally applied emulsion for treating dermatitis and eczema
InactiveCN1771969AAnti-inflammatoryAnti-eczemaHydroxy compound active ingredientsAerosol deliveryEthyl hydroxybenzoateAdditive ingredient
The present invention is externally applied emulsion for treating dermatitis and eczema, and the emulsion is prepared with clobetasol propionate in 1 weight portions and borneol in 30-50 weight portions as the effective components and through conventional preparation process. The material for the emulsion includes also excipient of octadecanol, stearic acid, glycerol monostearate, liquid paraffin, glycerin, HR-S1 emulsifier, emulsifying silica oil and ethyl hydroxybenzoate. Animal experiments show that the emulsion of the present invention has the functions of resisting inflammation, resisting eczema, stopping itch and relieving pain, and may be used in clinical experiment.
Owner:西安交大科技园博源生物医药有限责任公司
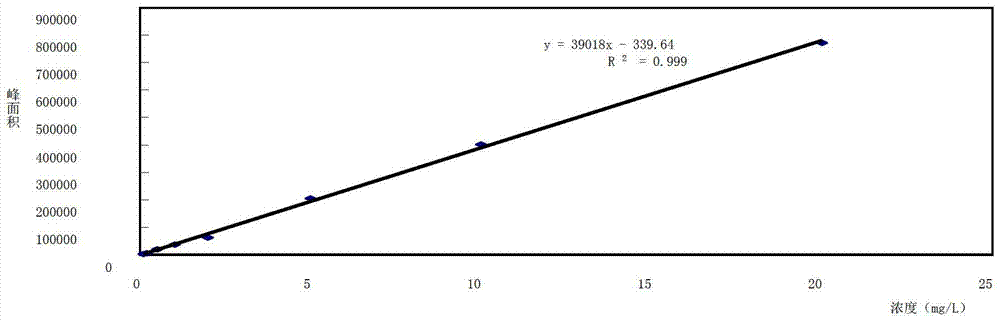
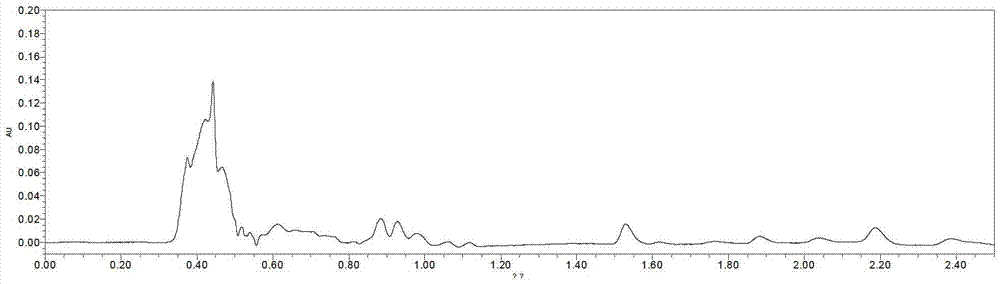
Method for measuring clobetasol propionate in products used for promoting growth and development of hairs
The invention discloses a method for measuring clobetasol propionate in products used for promoting the growth and the development of hairs; the method comprises the steps of: preparing a sample to be tested; carrying out ultra-high performance liquid chromatography measurement on the sample to be tested; adopting a BEH C18 (2.1*50mm, 1.7mu m) chromatographic column with the column temperature of 35-45 DEG C; taking acetonitrile and water as moving phases, and carrying out gradient elution; controlling the constant flow speed to be 0.3-0.4mL / min; and taking Waters personal digital assistant (PDA) as a detector, wherein the detection wavelength is 230-240nm. After being used for detecting the content of the clobetasol propionate in the products used for promoting the growth and the development of the hairs, the method is low in detection limit, high in degree of accuracy, simple in pretreatment and very suitable for the rapid batch detection of the clobetasol propionate in the products used for promoting the growth and the development of the hairs; and the method provides the important technical support for the quality control and safety monitoring of cosmetic products, and has good economic and social benefits.
Owner:GUANGZHOU QUALITY SUPERVISION & TESTING INST
Acne removing preparation
InactiveCN103536664AAcne Removal QuicklyAcne breakoutOrganic active ingredientsDermatological disorderSide effectKetoconazole
The invention provides an acne removing preparation with no side effect and good curative effect. The preparation comprises the following components in percentage by weight: 28-56 percent of ketoconazole and clobetasol propionate cream, 20-28 percent of lincomycin hydrochloride gel, 8-20 percent moroxydine hydrochloride, 4-10 percent of metronidazole and 10-40 percent of traditional Chinese medicinal extract, wherein the traditional Chinese medicinal extract comprises extract of white peony root, white poria, bighead atractylodes rhizome and angelica root. The acne removing preparation is effectively combined with traditional Chinese medicines and western medicines, can be used for quickly removing pox and acne, and has the effect of whitening.
Owner:朱晓明 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com