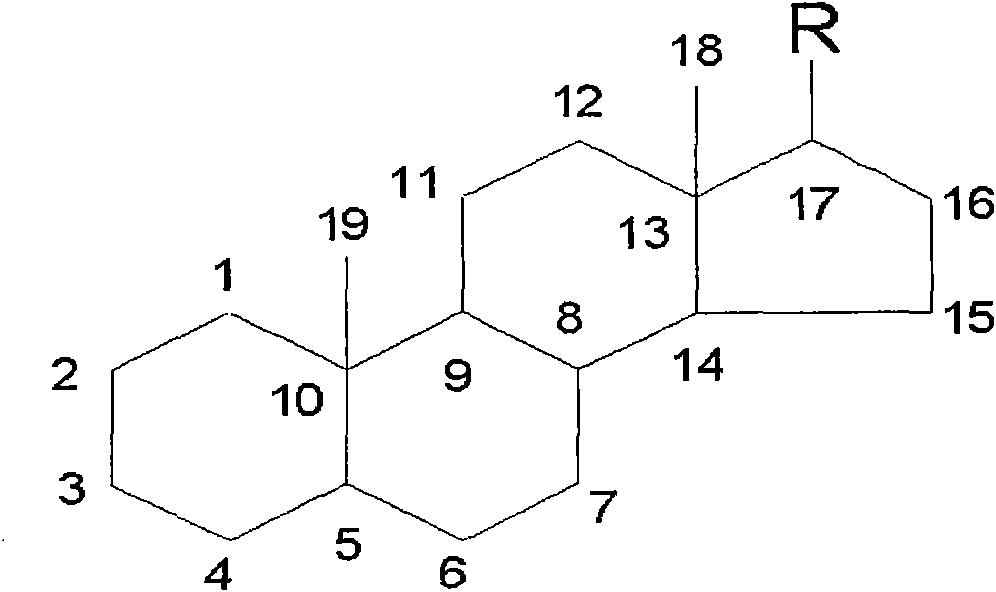
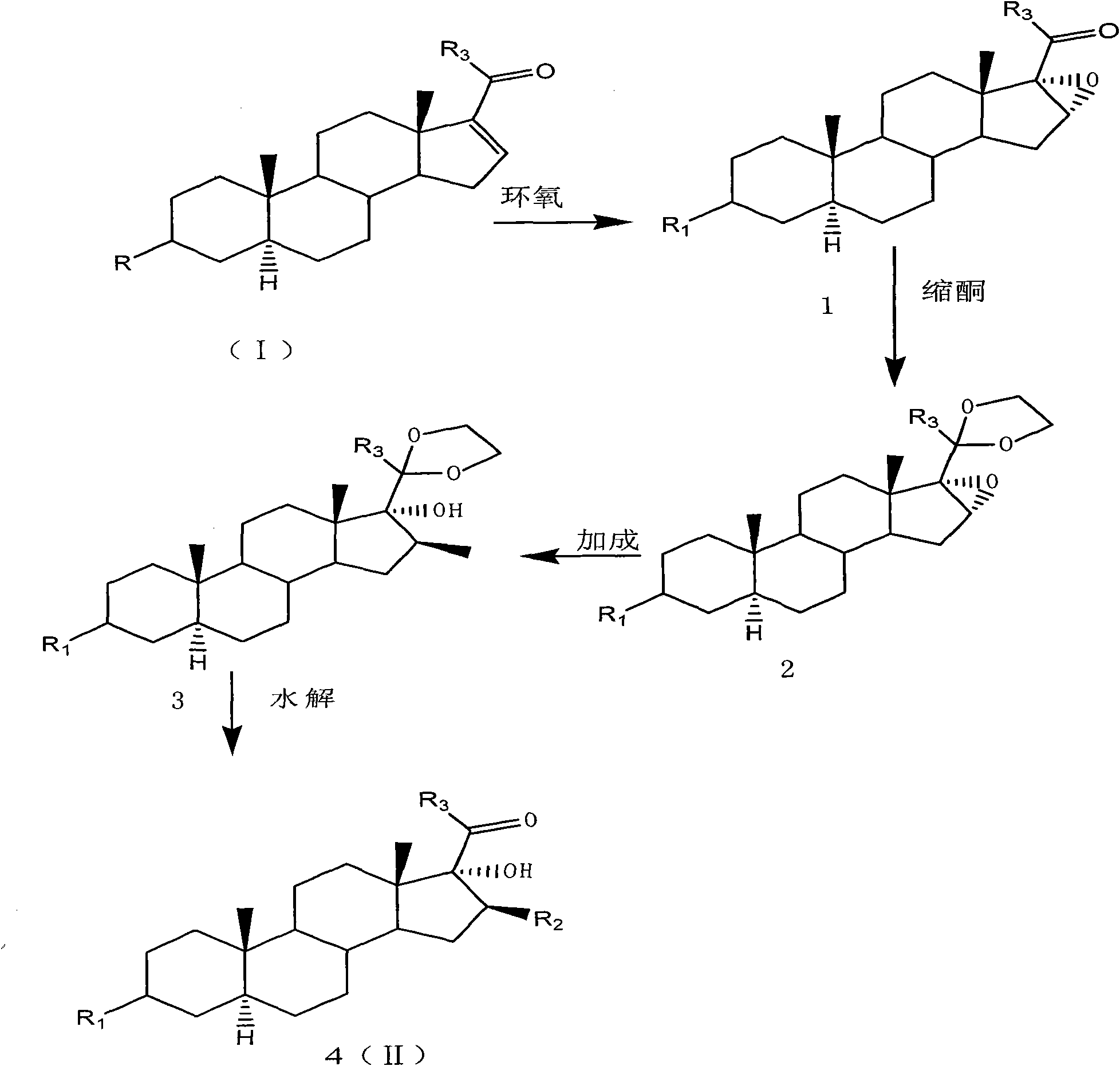
Preparation method of intermediate of steroidal drug with 16-beta-methyl
A technology of methyl steroids and intermediates, which is applied in the field of preparation of 16-β-methyl steroid drug intermediates, and can solve the problems of increasing the difficulty of refining and purifying in subsequent stages, difficult impurities, and affecting the quality of final products, etc. , to achieve the effect of easy access, high feasibility and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
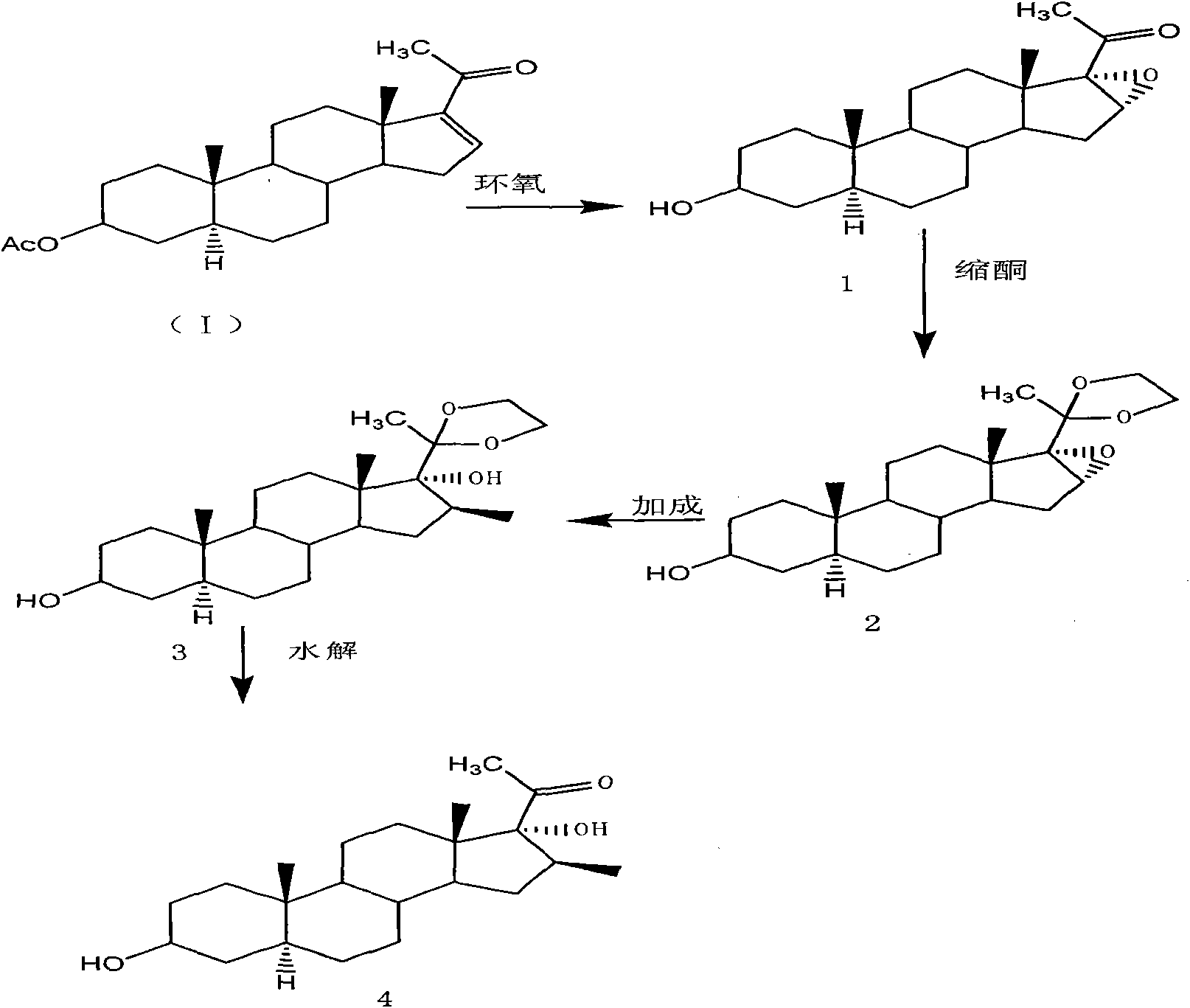
[0025] Preparation of pregna-16β-methyl-3β, 17a-diol-20-one from 5-a-pregna-16-ene-3β-ol-20-one-3-acetate, the reaction principle is as follows image 3 Shown, the specific reaction steps are as follows:
[0026] Epoxidation reaction:
[0027] Add 50g of 5a-pregn-16-en-3-ol-20-one-3-acetate (sisalomonene) into 500ml of methanol solvent, and stir to make it uniform. Control the temperature below 40°C, add 19ml H 2 o 2 , and 30 ml of 25% (w / w) NaOH solution. After the addition, stir and keep warm for 5 to 24 hours. After the reaction is complete, add sodium bisulfite solution (10%) under stirring until the starch-potassium iodide test paper is negative. Adjust the pH of the solution to 6-7 with acetic acid (appropriate amount). Add 350ml of water, then distill under reduced pressure until the reaction liquid is about 200ml, and cool to room temperature. The reaction solution was slowly poured into 600ml of cold water and stirred for 60min. Filter and dry the filter cake t...
Embodiment 2
[0035] Preparation of pregna-16β-methyl-3β, 17a-diol-20-one from 5-a-pregna-16-ene-3β-ol-20-one, the reaction principle is as follows Figure 4 Shown, the specific reaction steps are as follows:
[0036] Epoxidation reaction:
[0037] Add 25 g of 5-a-pregn-16-en-3β-ol-20-one into 250 ml of methanol and stir to make it uniform. Control the temperature below 40°C, add 10ml H2O2, and 15ml 25% (w / w) NaOH solution. After the addition, stir and keep warm for 5 to 24 hours. After the reaction is complete, add sodium bisulfite solution (10%) under stirring until the starch-potassium iodide test paper is negative. Adjust the pH of the solution to 6-7 with acetic acid (appropriate amount). Add 150ml of water, then distill under reduced pressure until the reaction liquid is about 100ml, and cool to room temperature. The reaction solution was slowly poured into 300ml of cold water and stirred for 60min. Filter and dry the filter cake to obtain 24.6 g of dry product (5a-pregna-16α-17α...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com