Method for synthesizing clobetasol propionate intermediate
A technology of clobetasol propionate and intermediates, which is applied in the field of synthesis of clobetasol propionate intermediates, can solve the problems of large solvent pollution, long process route, and many influencing factors, and achieve less environmental pollution and better Application prospects and the effect of improving the overall technical level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
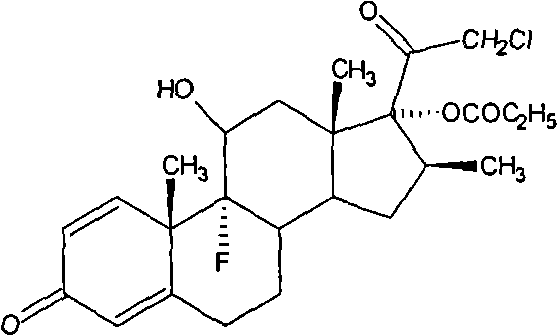
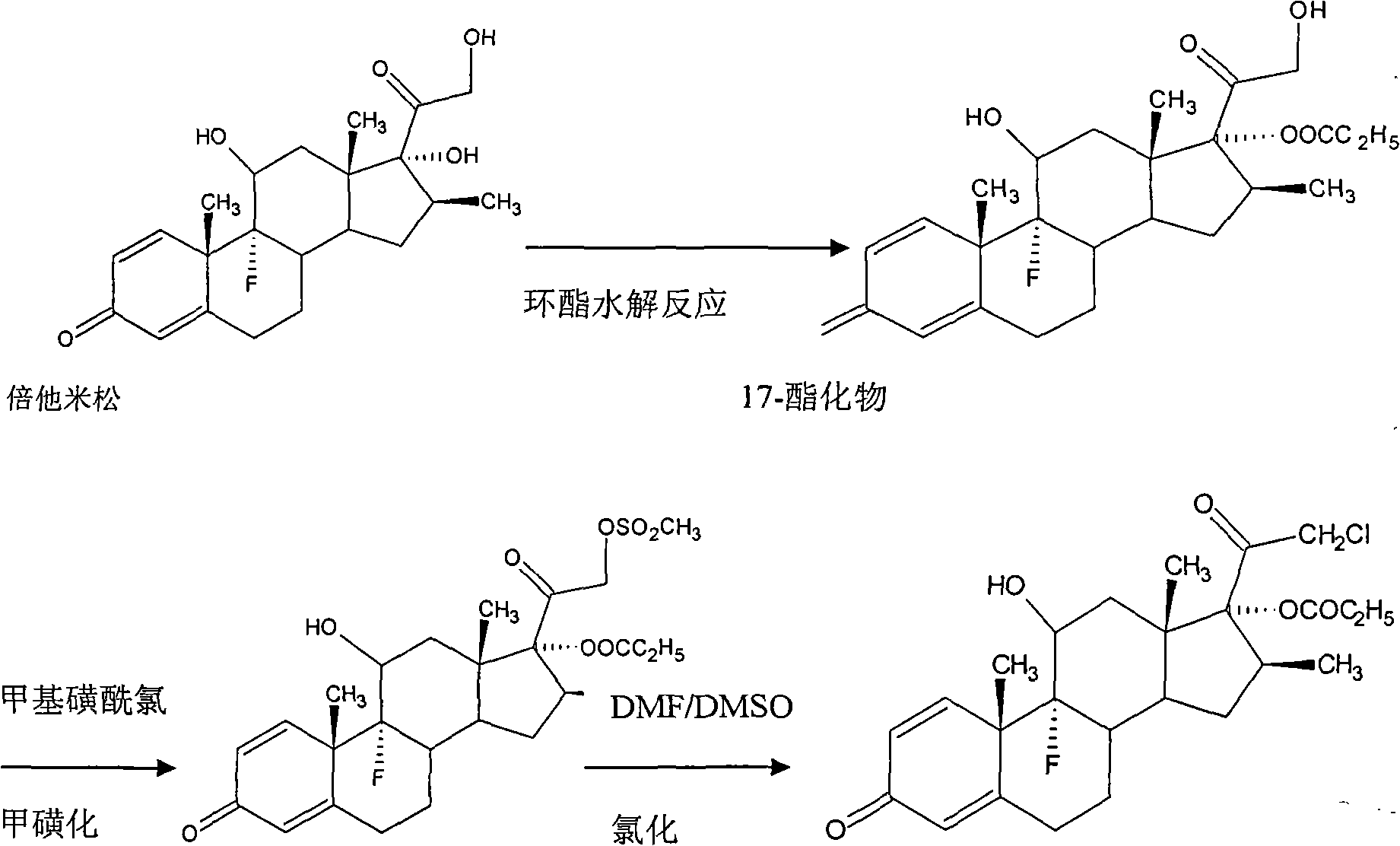
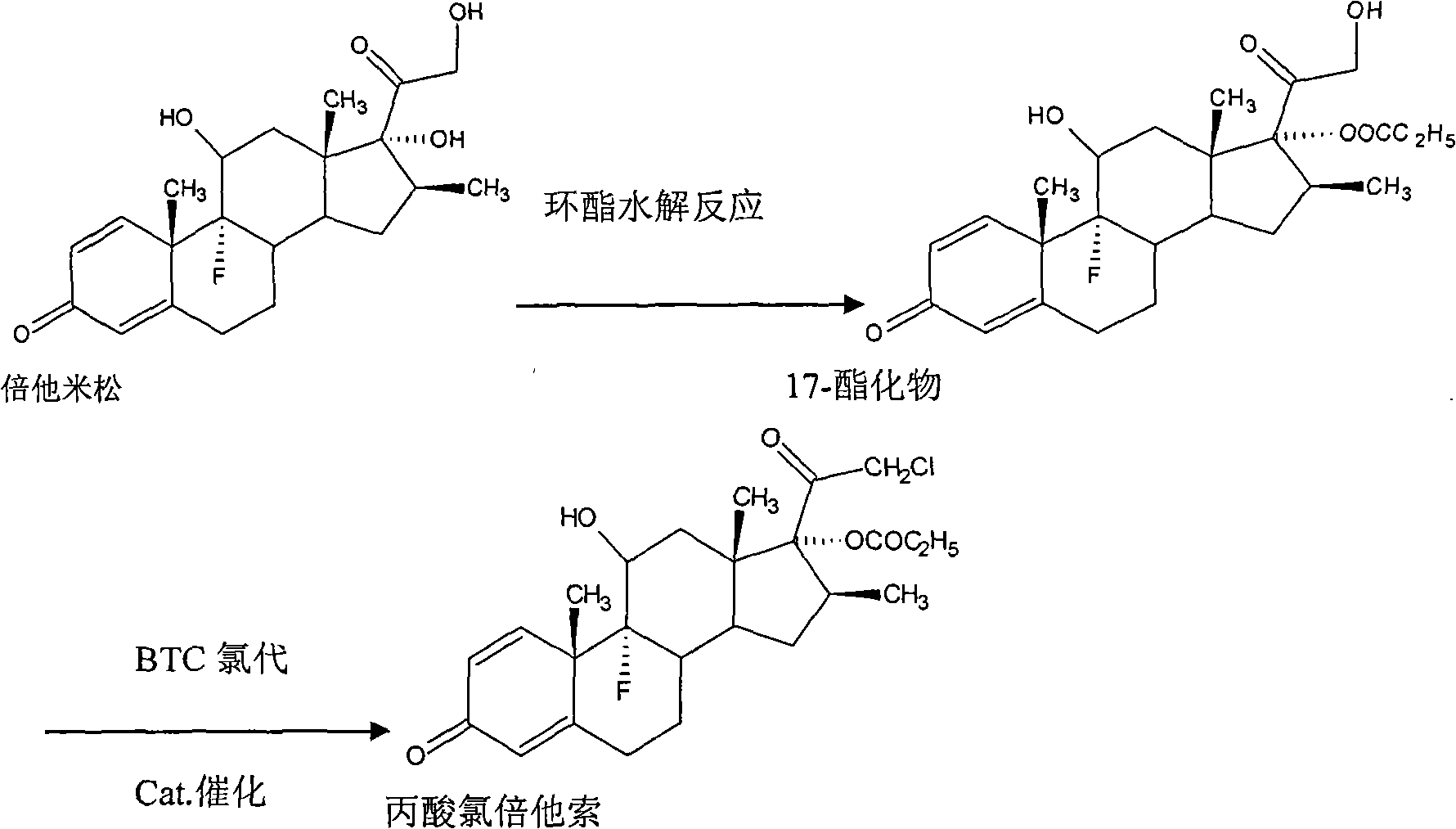
[0034] Dissolve 20 g of betamethasone 17-esterified product obtained by hydrolysis of betamethasone through cyclic ester in 150 ml of acetone, stir and dissolve fully, add 6 g of ZnCl 2 , after heating up to 35°C, add 30g of BTC. After passing through the BTC, keep it warm for 3 hours. After the reaction, concentrate under reduced pressure at a temperature of 40°C until the solution contains 30ml of acetone, and then add 300ml of acetone The drinking water was analyzed, filtered, and finally dried at a temperature of 80° C. for 16 hours to obtain 19.64 g of a crude product of clobetasol propionate. The yield was 98.2%, and the content of the crude clobetasol propionate was analyzed to be 96.9%.
Embodiment 2
[0036] Dissolve 20 g of betamethasone 17-esterified product obtained by hydrolysis of betamethasone through cyclic ester in 150 ml of acetone, stir and dissolve fully, then add 7.2 g of FeCl 3 , after heating up to 30°C, add 24g of BTC. After passing through the BTC, keep it warm for 2 hours. After the reaction, concentrate under reduced pressure at a temperature of 35°C until the solution contains 20ml of acetone, and then add 300ml The drinking water was analyzed, filtered, and finally dried at a temperature of 85° C. for 10 hours to obtain 19.5 g of a crude product of clobetasol propionate. The yield is 97.5%, and the crude product content of clobetasol propionate is 95.6% after analysis.
Embodiment 3
[0038] Dissolve 20 g of betamethasone 17-esterified product obtained by hydrolysis of betamethasone through cyclic ester in 150 ml of acetone, stir and dissolve fully, then add 4 g of AlCl 3After heating up to 35°C, 28g of BTC was added. After the BTC was passed through, the heat preservation reaction was carried out for 4 hours. After the reaction was completed, at a temperature of 30°C, concentrated under reduced pressure until the solution contained 20ml of acetone, and then added 300ml The drinking water was analyzed, filtered, and finally dried at a temperature of 75° C. for 18 hours to obtain 19.62 g of a crude product of clobetasol propionate. The yield is 98.1%, and the crude product content of clobetasol propionate is 95.8% after analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com