Compound clobetasol propionate mixed micellar solution and preparation method thereof
A technology of clobetasol propionate and compound chlorine propionate, which is applied in the field of biomedicine, can solve the problems of increased sample temperature, difficulty, and increased impurity content in the final product, and achieve the effect of increased drug retention and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
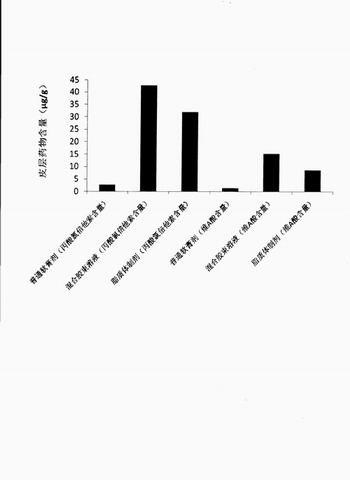
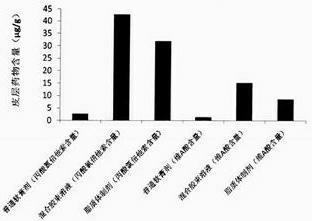
[0024] Embodiment 1: a kind of compound clobetasol propionate mixed micelle solution is characterized in that every 1000mg mixed micelle solution contains: clobetasol propionate 0.5mg, tretinoin 0.25mg, hydrogenated phosphatidylcholine 7.5mg mg, sodium cholate 22.5mg, and the balance is purified water.
[0025] Preparation process: take clobetasol propionate, tretinoin, hydrogenated phosphatidylcholine, sodium cholate, dissolve in 30 times the weight of ethanol; 50 ℃, rotary evaporate the organic solvent to obtain drug-mixed micelles co-precipitation Add an appropriate amount of purified water, oscillate and hydrate until the drug-mixed micelle co-precipitate film is completely dissolved, and the drug mixed micelle solution is obtained.
[0026] The encapsulation efficiency of clobetasol propionate in the obtained mixed micelle solution was 94.7%; the encapsulation efficiency of tretinoin was 98.7%; the average particle size was 30.7nm.
Embodiment 2
[0027] Embodiment 2: a kind of compound clobetasol propionate mixed micelle solution is characterized in that every 1000mg mixed micelle solution contains: clobetasol propionate 0.25mg, tretinoin 0.25mg, hydrogenated phosphatidylcholine 10mg , sodium cholate 40mg, and the balance is pH7.4 phosphate buffer.
[0028]Preparation process: Dissolve clobetasol propionate, retinoic acid, hydrogenated phosphatidylcholine, and sodium cholate in 20 times the weight of ethanol until dissolved; 50 ° C, rotary evaporate the organic solvent to obtain drug-mixed micelles Co-precipitate film: Add appropriate amount of pH 7.4 phosphate buffer, oscillate and hydrate until the drug-mixed micelle co-precipitate film is completely dissolved, and the drug mixed micelle solution is obtained.
[0029] The encapsulation efficiency of clobetasol propionate in the obtained mixed micelles solution was 93.2%; the encapsulation efficiency of tretinoin was 92.2%; the average particle size was 42.1nm.
Embodiment 3
[0030] Embodiment 3: a kind of compound clobetasol propionate mixed micelle solution is characterized in that every 1000mg mixed micelle solution contains: clobetasol propionate 0.25mg, tretinoin 0.25mg, phosphatidylethanolamine 10mg, remove Sodium oxycholate 40mg, the balance is pH7.4 phosphate buffer.
[0031] Preparation process: Dissolve clobetasol propionate, retinoic acid, phosphatidylethanolamine, and sodium deoxycholate in 40 times the weight of ethanol until dissolved; 50°C, rotary evaporate the organic solvent to dry up to obtain drug-mixed micelles Co-precipitate film: add an appropriate amount of pH7.4 phosphate buffer, oscillate and hydrate until the drug-mixed micelle co-precipitate film is completely dissolved, and the drug-mixed micelle solution is obtained.
[0032] The encapsulation efficiency of clobetasol propionate in the obtained mixed micelles solution was 85.1%; the encapsulation efficiency of tretinoin was 91.4%; the average particle size was 43.3nm. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average particle size | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com