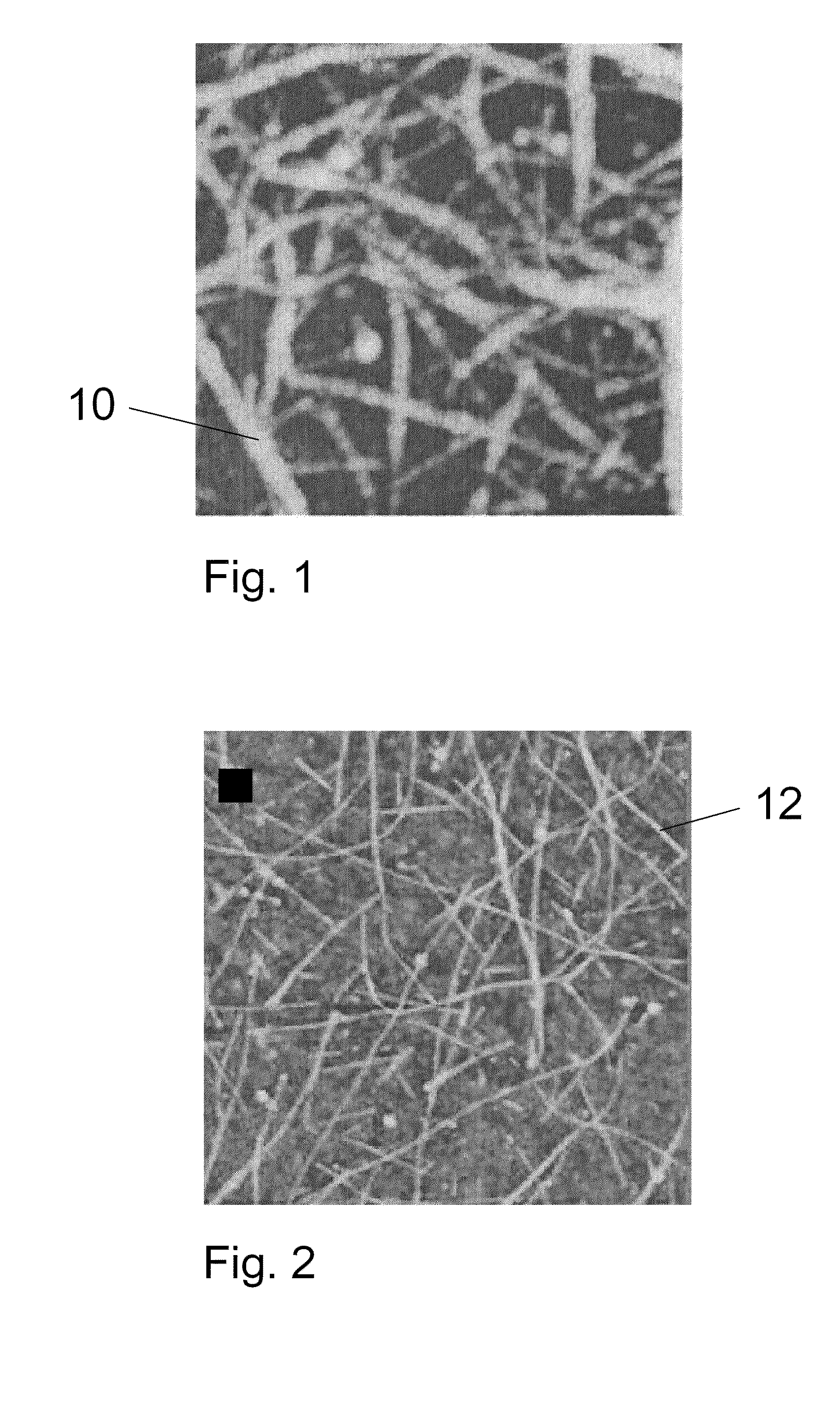
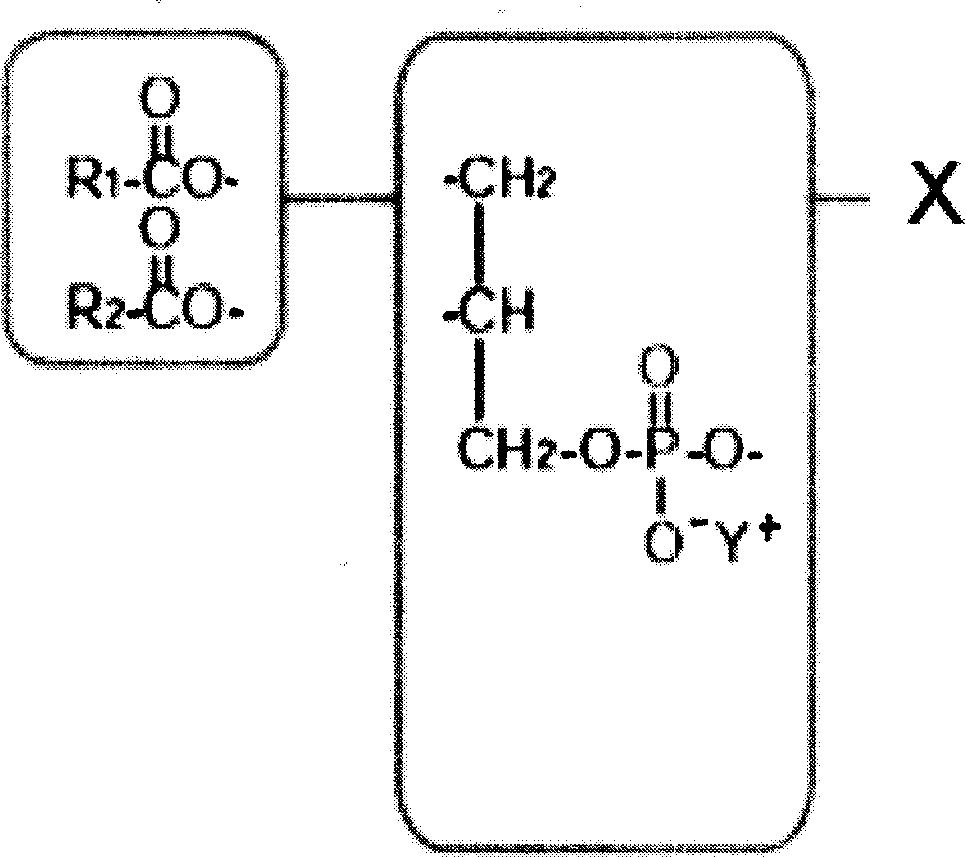
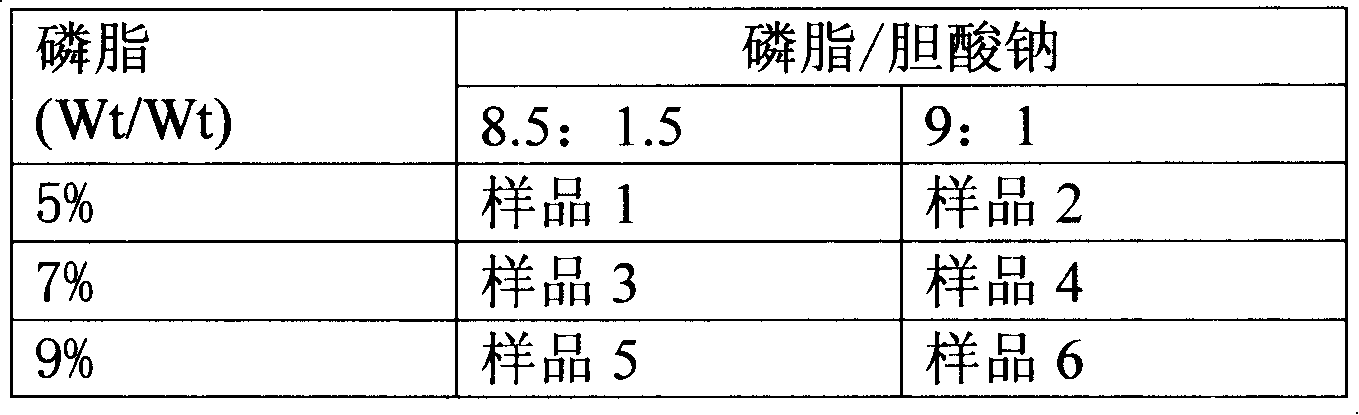
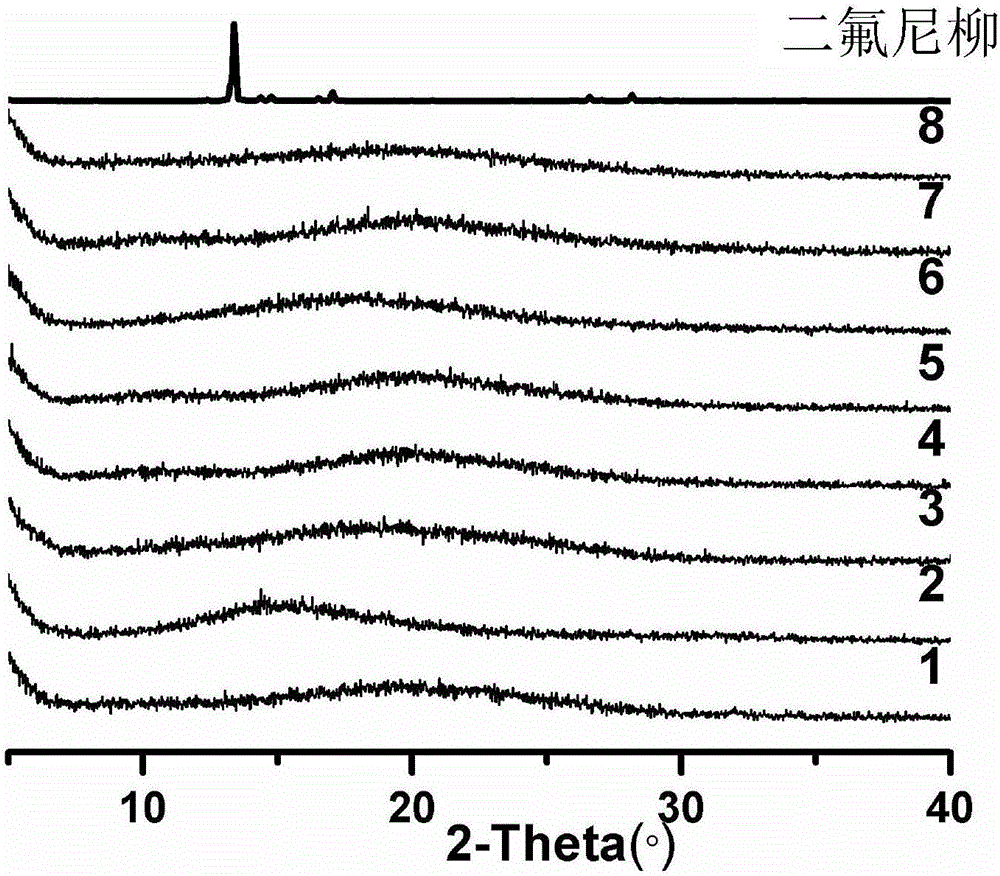
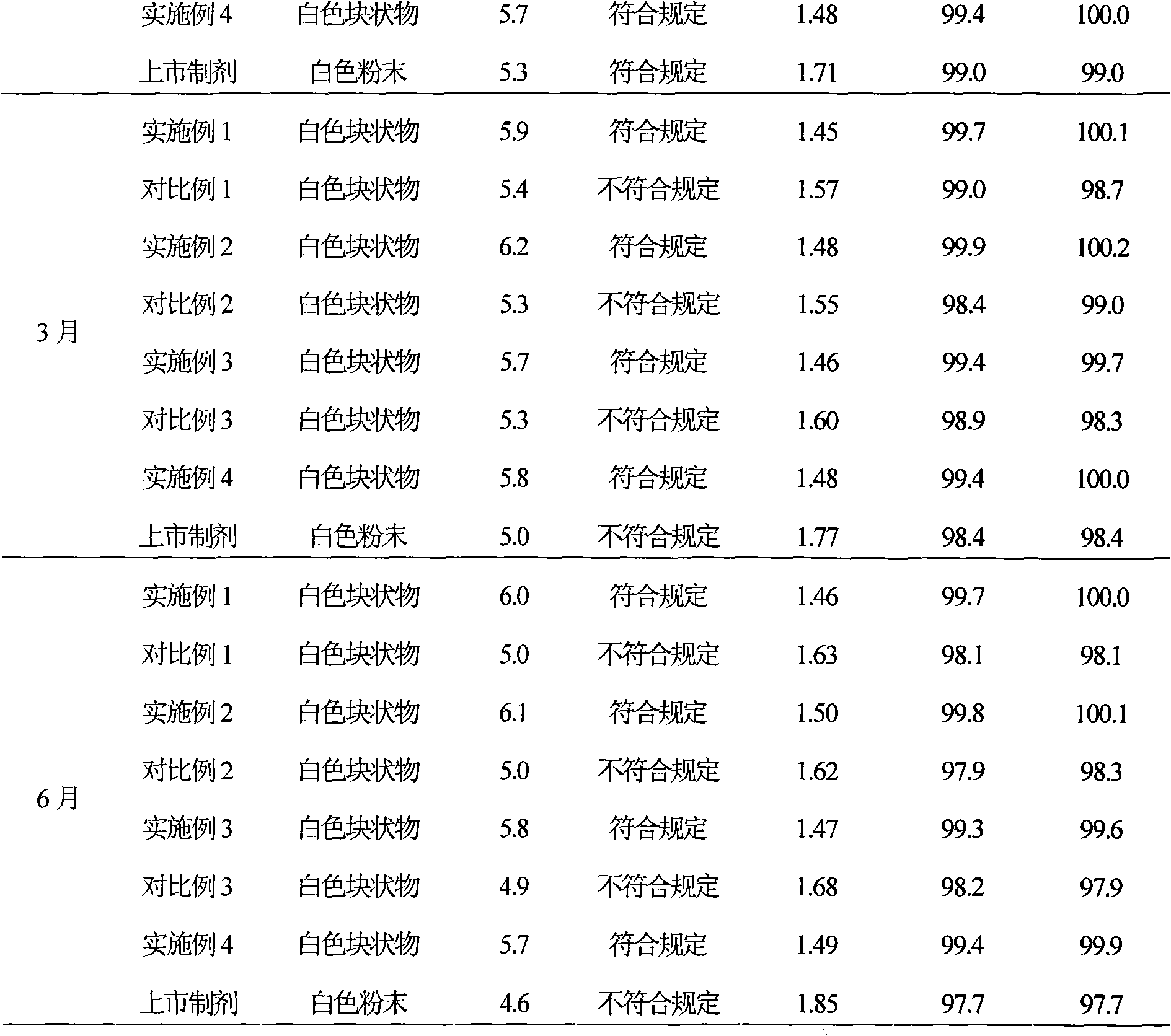
Patents
Literature
220 results about "Sodium Cholate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A trihydroxy bile salt that is used as a digestive aid in dietary supplements. It is used in culture media and in conjunction with PAPAIN and PANCREATIN.
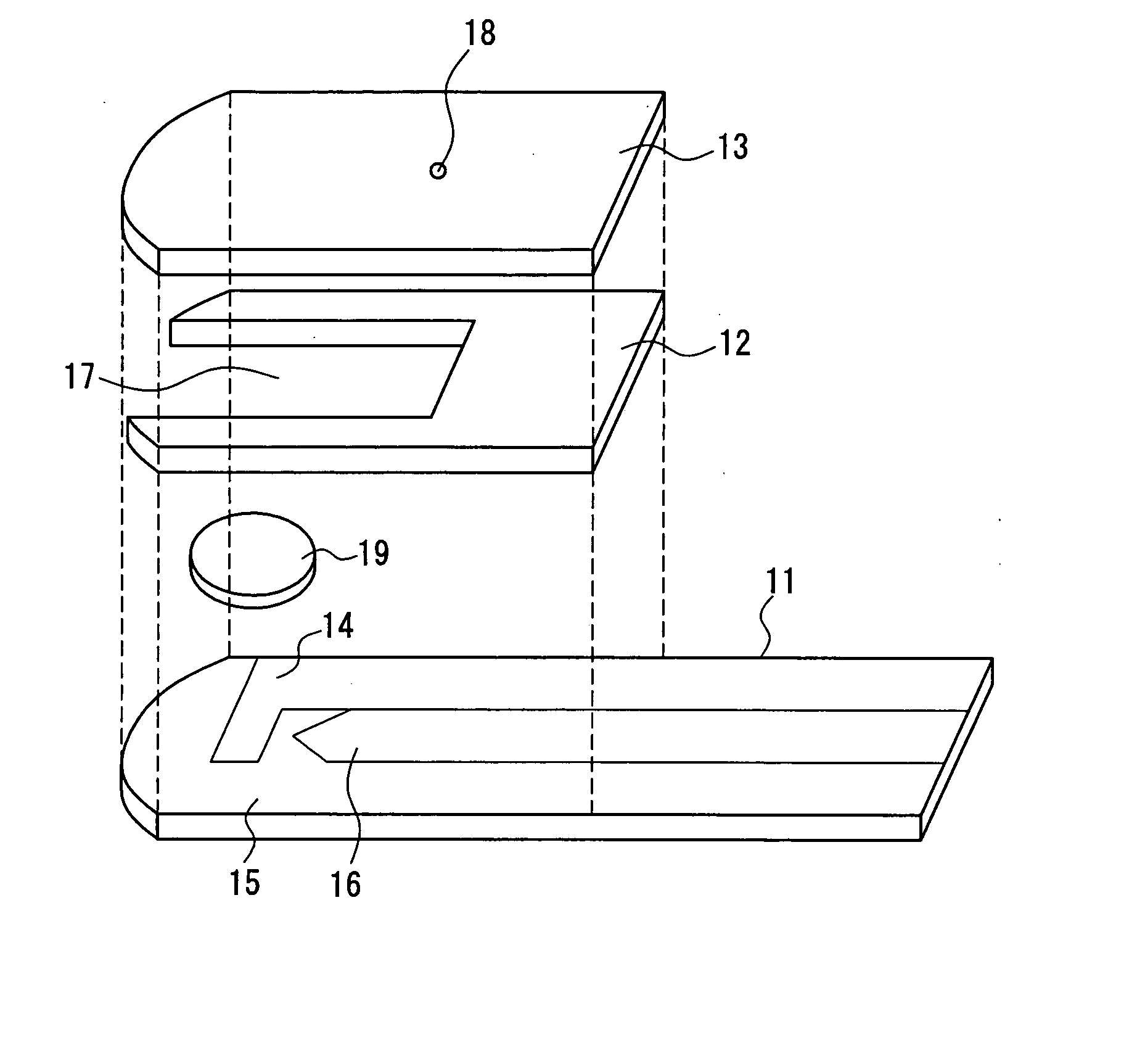
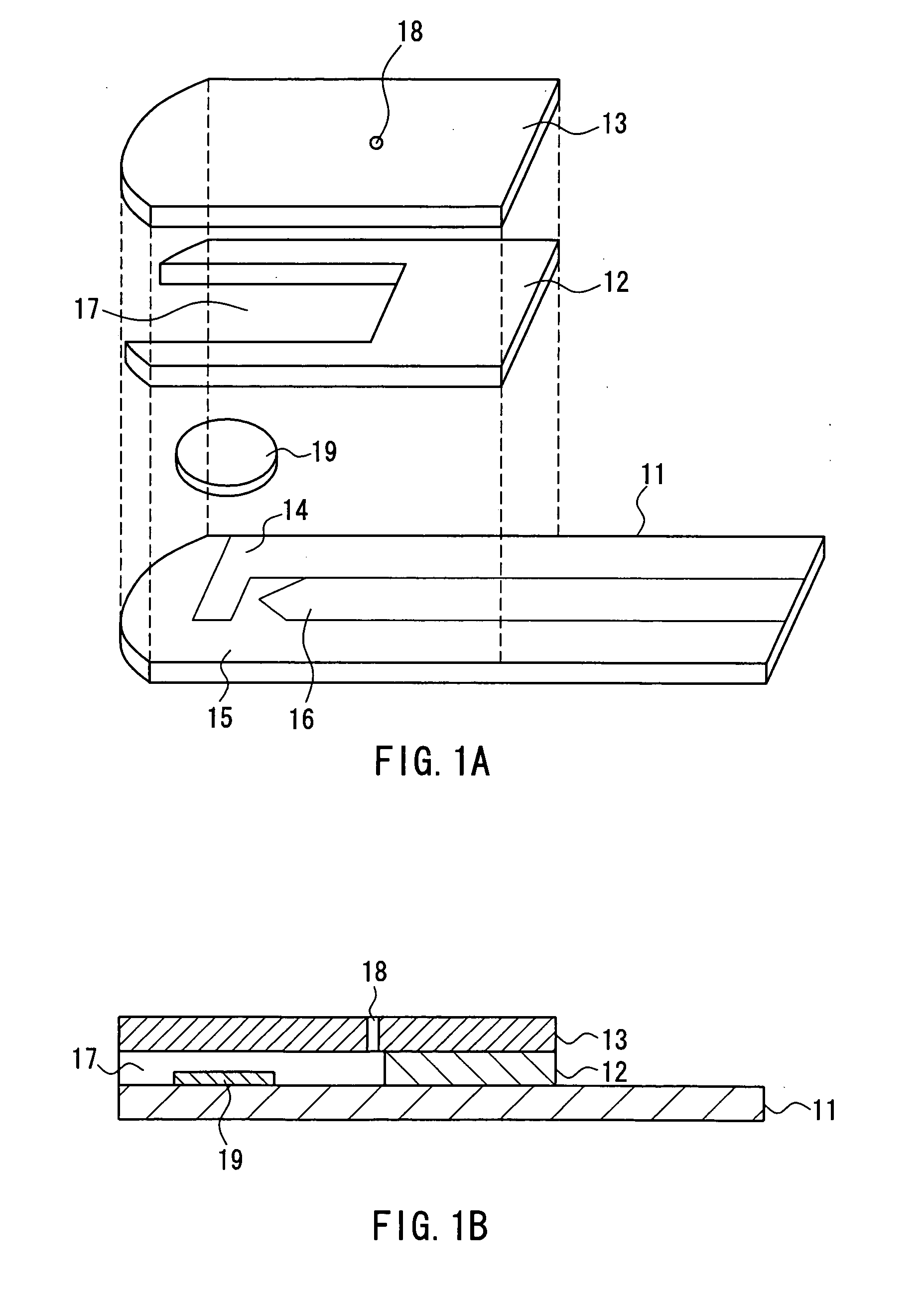
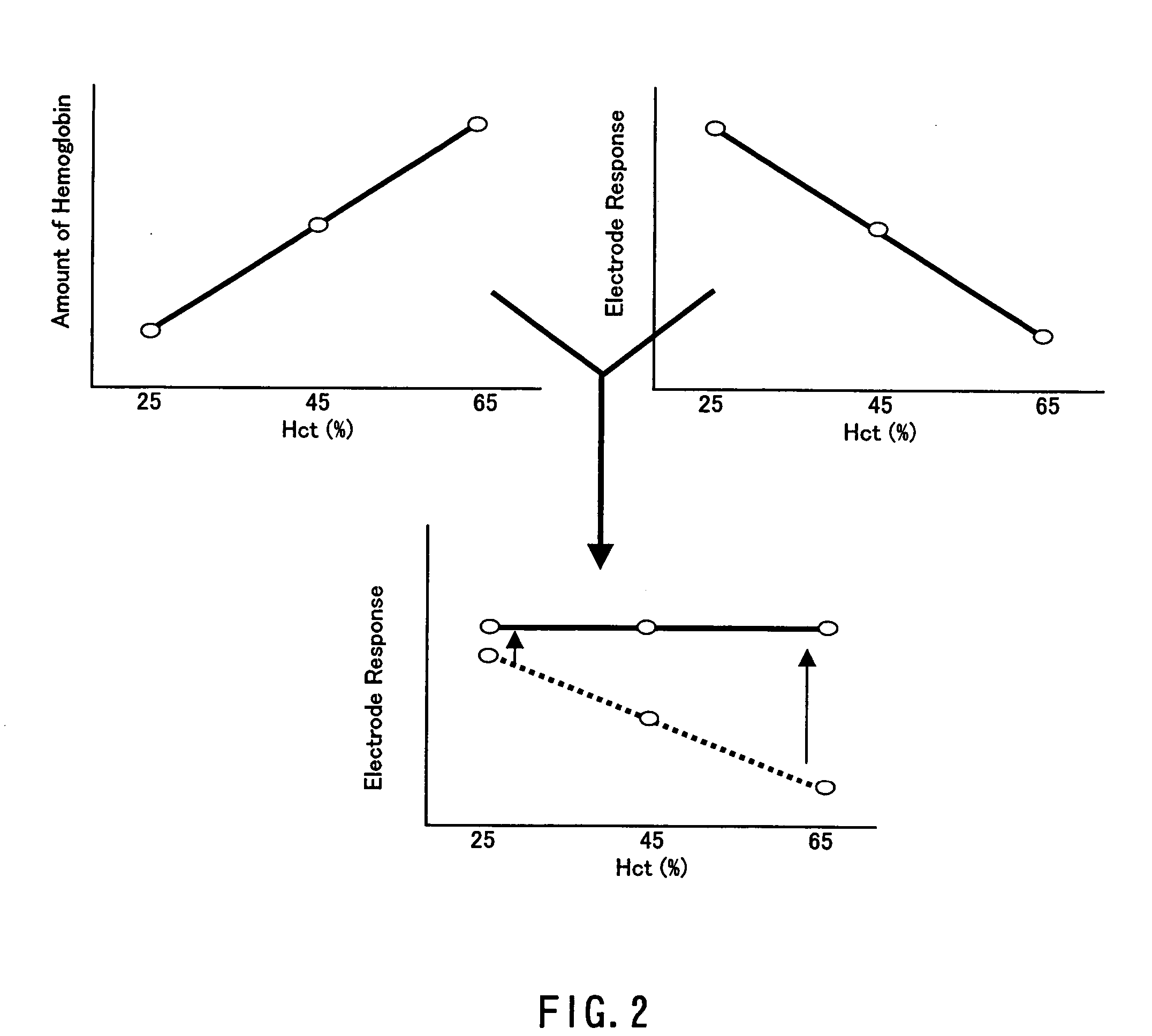
Method of measuring blood component and sensor used in the method
ActiveUS20050145490A1Great HctConvenient amountImmobilised enzymesBioreactor/fermenter combinationsRed blood cellOxidoreductase
A sensor for blood component analysis that can correct the effect of a hematocrit easily is provided. The sensor includes an analysis portion including a working electrode, a counter electrode, and a reagent portion. The reagent portion includes an oxidoreductase that reacts with the blood component and a mediator, and the blood component is measured by causing a redox reaction between the blood component and the oxidoreductase in the presence of the mediator and detecting a redox current generated by the redox reaction by the working electrode and the counter electrode. In this sensor, the reagent portion further includes a hemolyzing agent (e.g., sodium cholate) for hemolyzing an erythrocyte, and when detecting the redox current, the erythrocyte is hemolyzed with the hemolyzing agent so as to cause hemoglobin released to an outside of the erythrocyte to react with the mediator and a current generated by this reaction also is detected to correct an effect of a hematocrit.
Owner:PHC HLDG CORP
A kind of photovoltaic cell based on graphene pn junction and preparation method thereof
InactiveCN102290477AEasy to operateLow costFinal product manufacturePhotovoltaic energy generationCelluloseDoped graphene
The invention relates to a photovoltaic cell based on a graphene PN junction and a preparation method thereof. The photovoltaic cell is formed by a PN junction and a counter electrode, wherein the PN junction comprise a base, a transparent conductive thin film, a P type graphene thin film and a N type graphene thin film. The preparation method comprises the following steps of: cleaning the base and the transparent conductive thin film, and blow-drying the base and the transparent conductive thin film by using N2 for standby application; respectively preparing boron doped graphene and nitrogen-doped graphene; respectively dissolving the two prepared graphene in sodium cholate hydrate to prepare a graphene solution; carrying out suction filtering on a nitrogen-doped graphene solution by using a mixed cellulose filter membrane, dropping deionized water, slowly dropwise adding a boron doped graphene solution on the surface of a nitrogen-doped graphene thin film for suction filtering and film formation, inversely arranging the obtained thin film on the surface of the spare base, compacting, distilling by adopting acetone, and soaking and cleaning by sequentially using acetone and methyl alcohol under a room temperature condition; and covering the counter electrode on the surface of the counter electrode and pressing to form the graphene PN junction photovoltaic cell. The method is simple in the preparing and assembling process, is low in cost, is suitable for large-scale application and promotes the application of the graphene in terms of the solar battery.
Owner:QINGDAO UNIV OF SCI & TECH
Biodegradable nanoparticle-entrapped oral colon-targeted micro-capsule and preparation method thereof
ActiveCN103417515AObvious pH responseSignificant pH response characteristicsPharmaceutical non-active ingredientsMicrocapsulesOral medicationPolyvinyl alcohol
The invention discloses a biodegradable nanoparticle-entrapped oral colon-targeted micro-capsule and a preparation method thereof. The preparation method comprises the following steps: 1, dissolving drugs and degradable polymers in an organic phase and taking a solution containing polyvinyl alcohol or sodium cholate as a water phase for preparing drug nanoparticles; 2, dissolving an enteric-coated material in absolute ethanol; 3, dispersing the drug nanoparticles in absolute ethanol in which the enteric-coated material is dissolved; 4, preparing edible oil; 5, dropwise adding ethanol liquid into the edible oil, stirring, solidifying, and volatilizing to remove absolute ethanol; 6, centrifuging suspension liquid, collecting the micro-capsule in a lower layer, and washing by normal hexane. The prepared oral colon-targeted micro-capsule has a remarkable pH response, drugs in the micro-capsule are hardly released under the acidic conditions, a capsule material is dissolved under the pH value of enteric canal, the drug nanoparticles are released, the nanoparticles can be transferred to a target point, and the drugs are slowly released, so that the absorption rate of oral administration is greatly increased, the bioavailability is improved, and the treatment effect is enhanced.
Owner:SUN YAT SEN UNIV
Process for cleaning carbon nanotubes and other nanostructured films
InactiveUS20150202662A1Increase the washing processMaterial nanotechnologyNon-surface-active detergent compositionsActive agentPhysical chemistry
A process for the cleaning of carbon nanostructure and similar materials and structures for removal of surfactant chemicals. The process includes washing the carbon nanostructures with concentrated acetic acid which may be glacial acetic acid. The cleaning process is also considered in carbon nanostructure film preparation with deposition of carbon nanostructures in solution with surfactant chemicals before the washing. Possible surfactants include sodium cholate (SC) and sodium dodecyl sulfate (SDS). Carbon nanostructure deposition on a substrate may be by various printing methods.
Owner:ANEEVE LLC DBA ANEEVE NANOTECH
Freeze dried polyene lecithin powder for injection and its prepn
ActiveCN1771914AImprove solubilityImprove drug activityPowder deliveryOrganic active ingredientsPolyene phosphatidylcholineSodium dehydrocholate
The present invention discloses one kind of freeze dried polyene phosphatidylcholine powder for injection and its preparation process. It features that the preparation contains polyene phosphatidylcholine and solubilizer sodium deoxycholate, sodium dehydrocholate or sodium cholate in the weight ratio of 0.5-1.5.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Elastic nano vesicle preparations containing paclitaxel or docetaxel and preparation thereof
InactiveCN101209251AOrganic active ingredientsPharmaceutical non-active ingredientsDocetaxelPhosphate
The invention pertains to the technical field of medicine, which more particularly relates to an elastic nano-vesicle preparation used for transitting the active ingredient paclitaxel (taxol) or docetaxel to penetrate the natural permeability barriers or pores (such as, skin, mucosa, tissues and organs, etc.) and a preparation method thereof. The preparation at least contains the following components with the weight percentages: 3 to 15 percent of phospholipid, 0.3 to 5 percent of sodium cholate or sodium deoxycholate, 0.01 to 5 percent of paclitaxel or docetaxel, 5 to 30 percent of alcohol and the rest of water or phosphate buffer solution. The formed elastic nano-vesicle has lipid double molecular layers, and the diameter of the typical elastic nano-vesicle is less than 200nm. The preparation is used for the treatment of malignant tumors, and the invention can be taken by injection, spray or transdermal drug delivery.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Solid dispersion as well as preparation method and application thereof
ActiveCN106420633AImprove solubilityImprove bioavailabilityPharmaceutical non-active ingredientsGranular deliveryAlpha-TocopherolPolyethylene glycol
The invention relates to solid dispersion as well as a preparation method and an application thereof. The solid dispersion is prepared from indissolvable drugs, a surfactant and a water-soluble polymer material with a spray drying method after mixing and heating dissolution, wherein the surfactant is selected from at least one of sodium dodecyl sulfate, poloxamer, tween, alpha-tocopherol, succinate, polyethylene glycol, sodium cholate and polyethylene glycol / vinyl caprolactam / vinyl acetate copolymer; the water-soluble polymer material is selected from at least one of povidone, copovidone, hydroxypropyl methylcellulose and polyethylene glycol. An organic solvent is not required when the solid dispersion is prepared with the spray drying method, and the problem of organic solvent residues is solved. By means of the solid dispersion, the dissolvability of the indissolvable drugs is increased, the dissolution speed and the dissolubility are remarkably increased, and the bioavailability of the indissolvable drugs is improved.
Owner:GUANGZHOU ZHONGDA NANSHA TECH INNOVATION IND PARK +1
Suspensoid powder injection of amoxicillin sodium sulbactam sodium medicine composition and novel application thereof
InactiveCN101632660AUnexpected effectImprove stabilityPowder deliveryPharmaceutical product form changeActive componentFreeze-drying
The invention relates to suspensoid powder injection of an amoxicillin sodium sulbactam sodium medicine composition and a preparation method thereof. The suspensoid powder injection contains Tween 80, cholesterol, deoxysodium cholate, a freeze-drying support agent and active components. The invention also relates to a novel application of the suspensoid powder injection in preparing a medicine treating prostatitis further.
Owner:HAINAN YONGTIAN PHARMA INST
Oral solid lipid nano-particle preparation of calcitonin and preparation method thereof
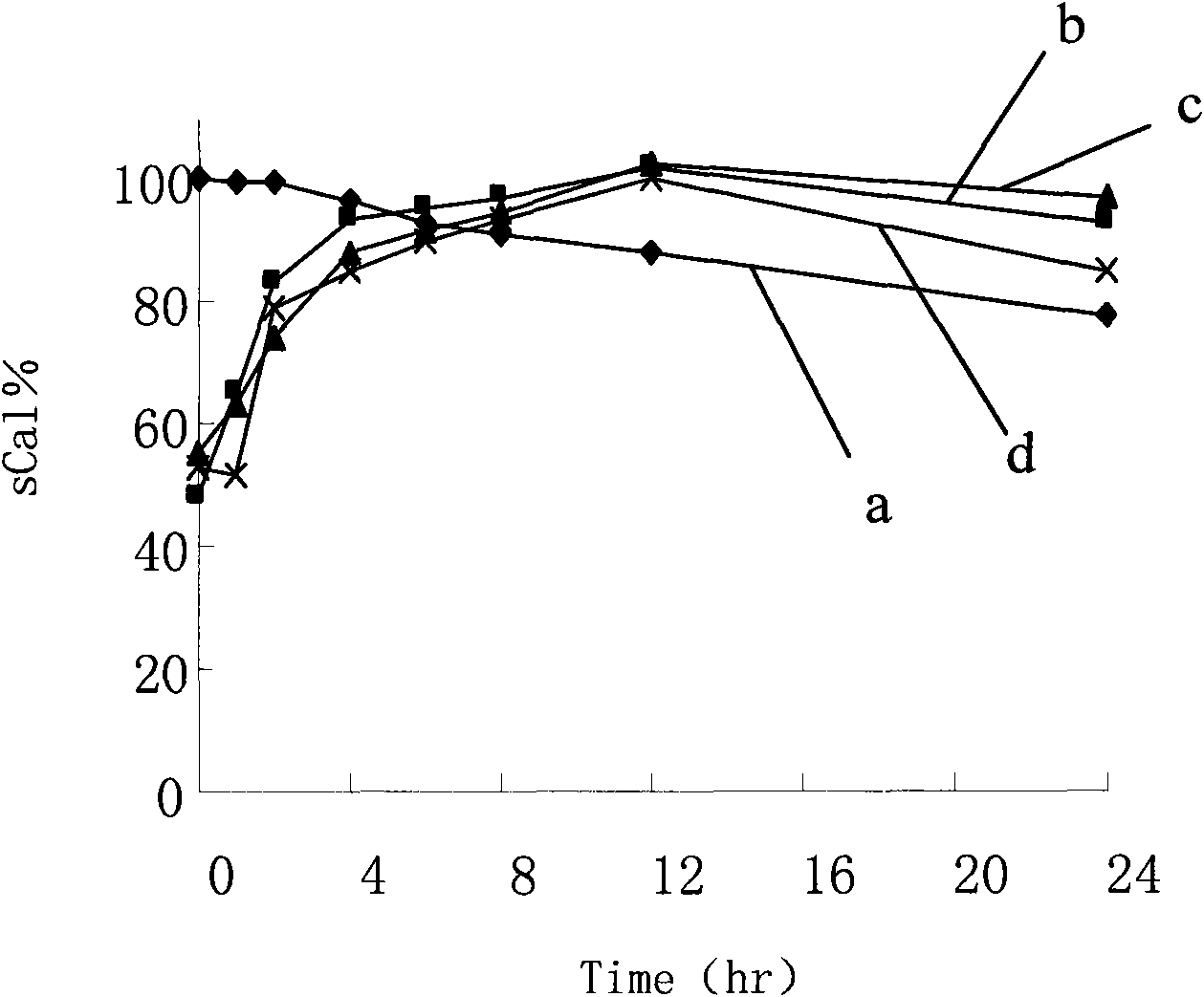
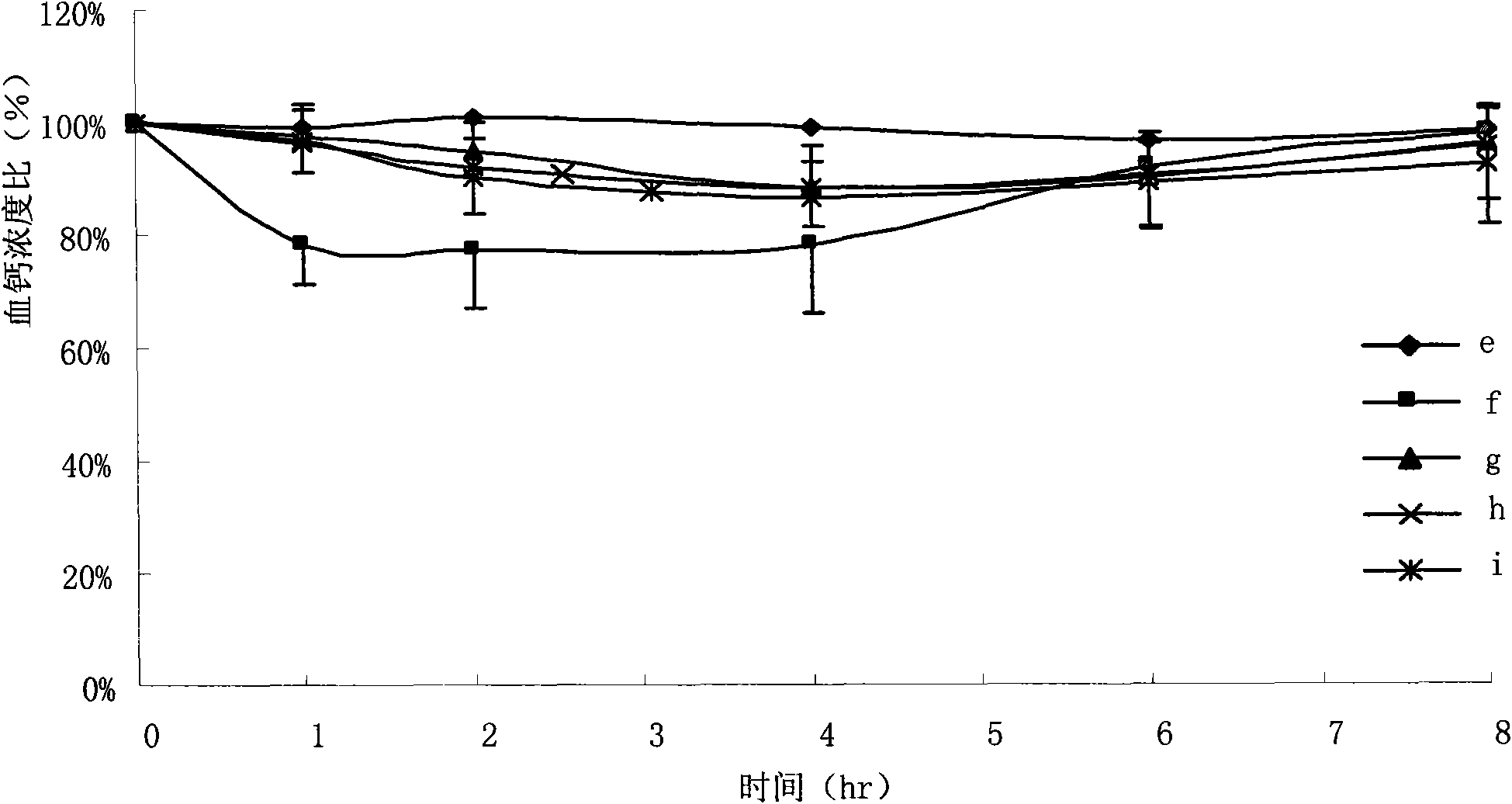
ActiveCN101569608AReasonable blood calcium concentrationPeptide/protein ingredientsSolution deliveryLipid formationMicroparticle
The invention discloses an oral solid lipid nano-particle preparation of calcitonin, which is a particle suspension containing 0.01 percent sodium cholate in a water phase, wherein lipid nano-particles comprise an active medicament component and a lipid material forming the particles. Simultaneously, the preparation method comprises the following steps: dissolving or melting a medicament and a carrier in an organic solvent phase together; quickly infusing the organic phase into the water phase stirred at a low temperature to form a lipid nano-particle dispersion liquid; standing, melting and performing high-speed centrifugal separation on the lipid nano-particle dispersion liquid to obtain a nano-particle deposition; and dispersing the deposition to obtain a target oral calcitonin lipid nano-particle dispersion liquid. The oral solid lipid nano-particle preparation uses the lipid material as a structural matrix material, and the oral solid lipid nano-particle preparation of the calcitonin is prepared through reasonable component proportion to prevent the medicament from leaking in an aqueous medium and release the medicament in modes of in vivo esterase degradation and the like, thus the aim that the medicament contained in a nano-carrier can adjust the serum calcium concentration more reasonably and more safely through biomembranes of mammals is achieved.
Owner:上海宝龙药业股份有限公司
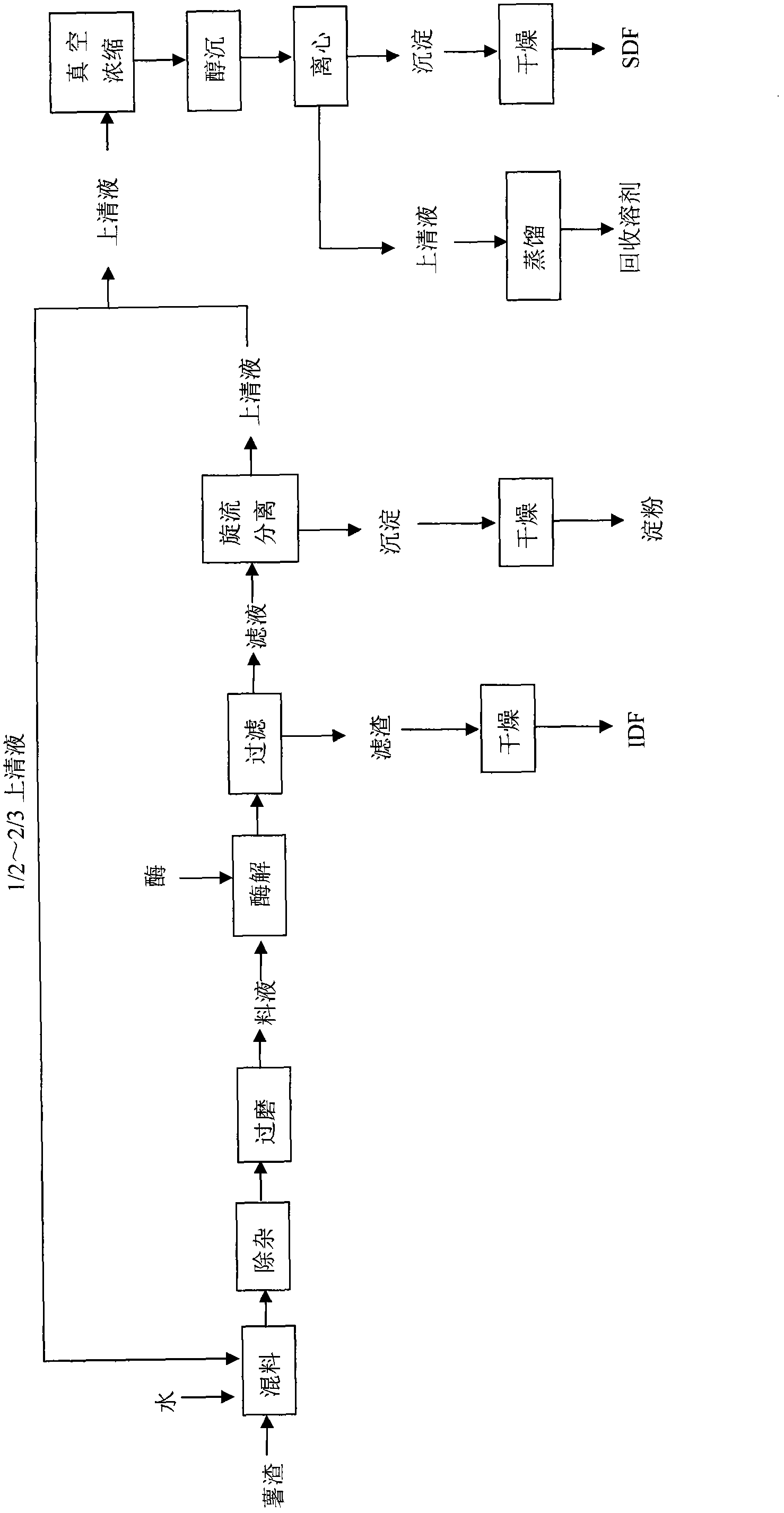
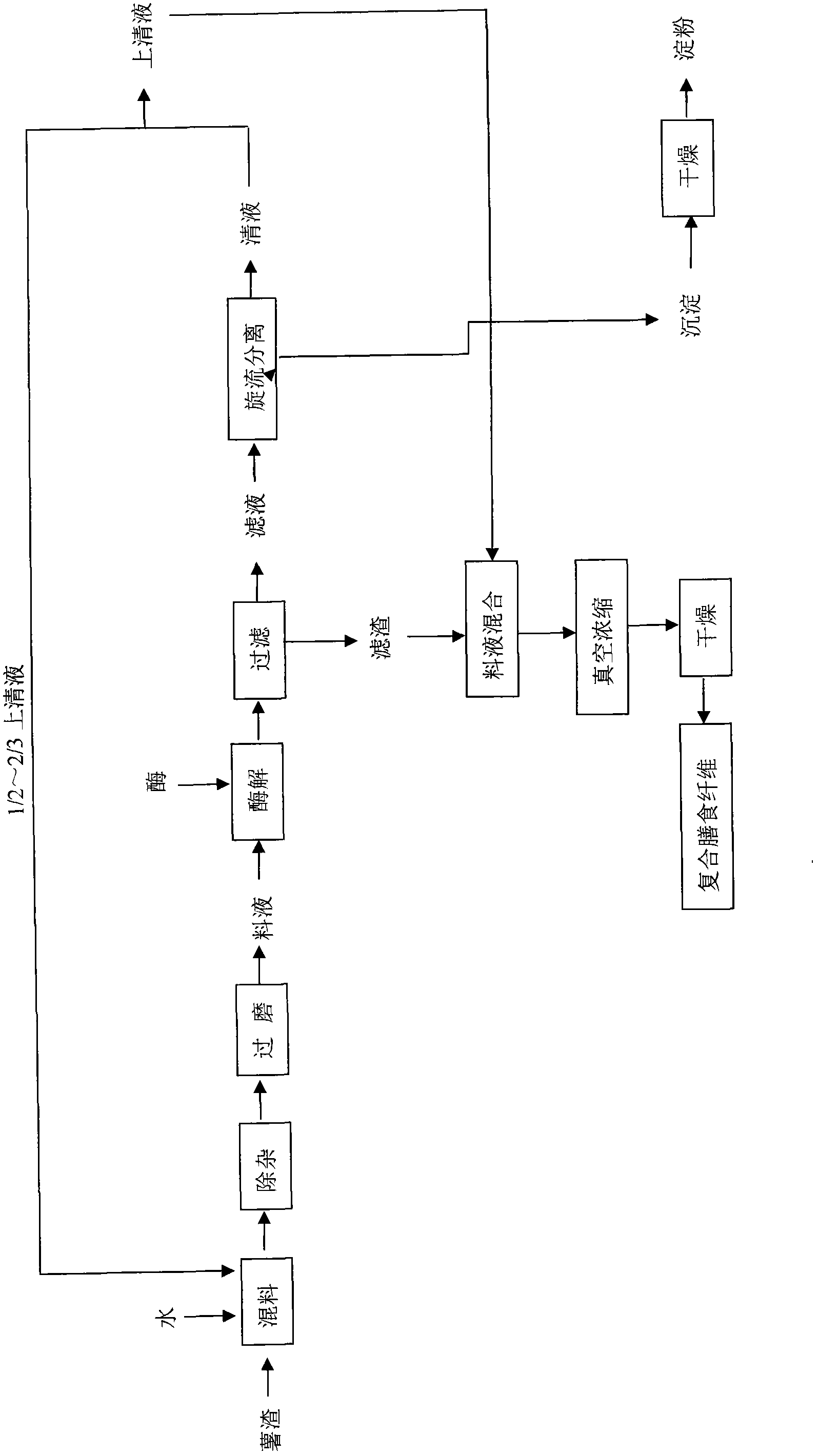
Potato slag comprehensive utilization processing method for combined production of starch and meal fiber
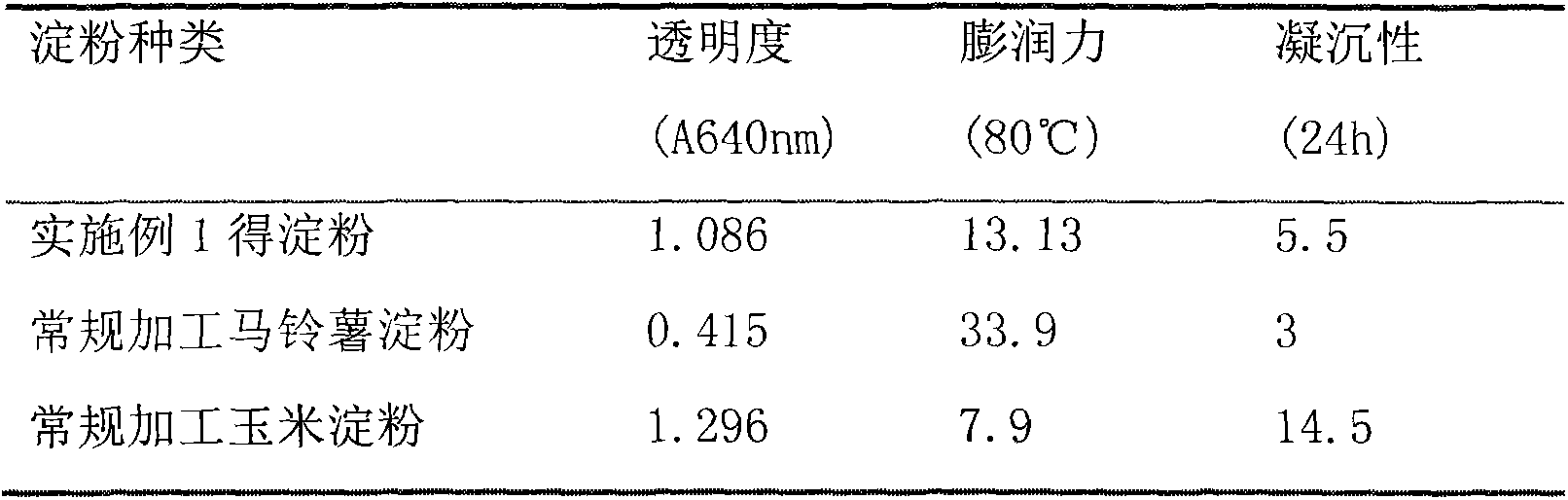
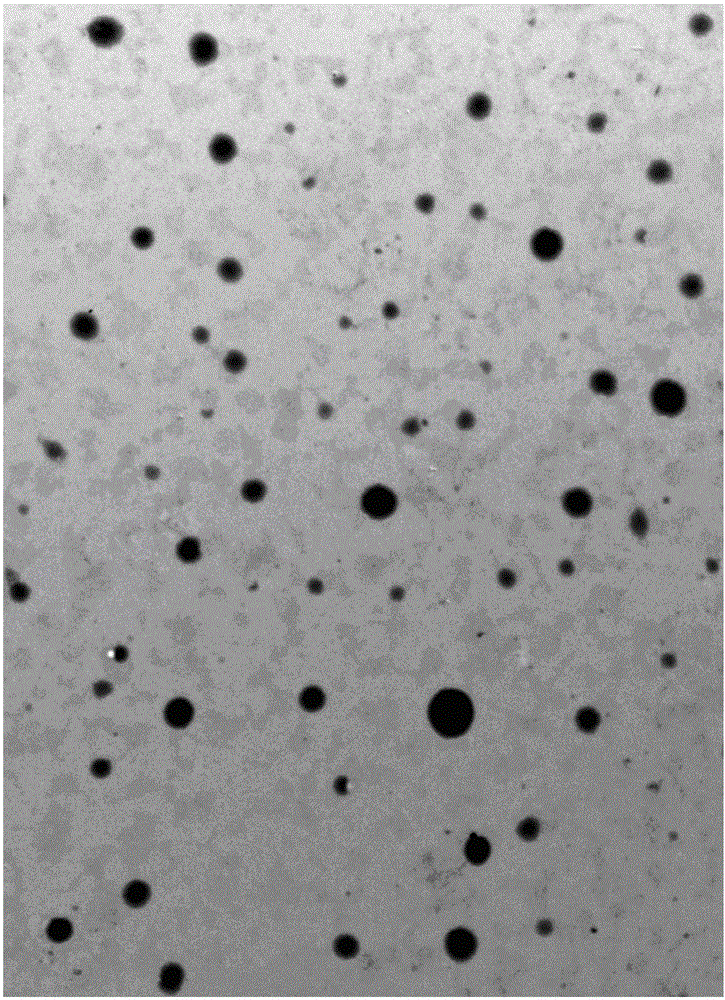
The invention discloses a potato slag comprehensive utilization processing method for combined production of starch and meal fiber. Mechanical crushing and enzyme degradation method are used for processing potato slag, so as to significantly improve the processing properties of materials, and effectively promote the release of target and generation of high active components. At the same time, medium recycling utilization process is utilized, so as to not only save water consumption but also simplify the subsequent concentration process, and effectively realize enrichment of target object. The obtained starch product has good transparency, swelling power and retrogradation property compared with other starch; and the obtained dietary fiber product has high proportion of soluble dietary fiber, strong sodium cholate, and especially obvious advantages in the aspects of adsorption ability of cholesterol adsorption compared with similar products, which shows that the dietary fiber product has good physical property and physiological activity. The invention has the advantages of simple operation, convenience, mild conditions, low equipment requirement, high utilization rate of raw materials, energy saving and benefit for environmental protection.
Owner:CHINA NAT RES INST OF FOOD & FERMENTATION IND CO LTD
Flexible nano-liposome freeze-dried powder prepared by cistanche tubulosa extract phenylethanoid glycosides and preparation method thereof
ActiveCN105106024APromote transdermal absorptionProlong the action timeCosmetic preparationsToilet preparationsCholesterolLiposome
The invention relates to the technical field of flexible nano-liposome freeze-dried powder and a preparation method thereof, in particular to flexible nano-liposome freeze-dried powder prepared by cistanche tubulosa extract phenylethanoid glycosides and a preparation method thereof. In preparation, the flexible nano-liposome freeze-dried powder prepared by cistanche tubulosa extract phenylethanoid glycosides comprises the raw materials of the cistanche tubulosa extract phenylethanoid glycosides, lecithin, cholesterin and sodium cholate. Cistanche tubulosa extract phenyylethanoid glycosides are prepared into the nano-liposome freeze-dried powder for the first time, the transdermal absorption effect of cistanche tubulosa extract phenylethanoid glycosides is improved, meanwhile, the action time of cistanche tubulosa extract phenylethanoid glycosides is prolonged, and according to the features that the flexible nano-liposome freeze-dried powder prepared by cistanche tubulosa extract phenylethanoid glycosides has good encapsulation efficiency and is small in particle size and good in stability, technical bases are provided for development of cosmetics or related products with cistanche tubulosa extract phenylethanoid glycosides being main components at the later period.
Owner:THE FIRST TEACHING HOSPITAL OF XINJIANG MEDICAL UNIVERCITY
Polyene phosphatidyl choline lyophilized powdered injection
The invention discloses a polyene phosphatidyl choline freeze dried injection for the treatment of hepatic diseases, which comprises polyene phosphatidyl choline as the main ingredients and pharmaceutically acceptable carrying agents, wherein the carrying agent can be auxiliary solvent selected from sodium cholate, sodium deoxycholate, sodium deoxycholate, hydroxypropyl-beta-cyclodextrin, hydroxyethyl-beta-cyclodextrin and / or methyl cyclodextrin. The injection has higher stability.
Owner:GUANGDONG XIANQIANG PHARMA
Preparation method of micro-emulsion kit
ActiveCN102621138AImprove stabilityImprove the problem of large difference between batchesMaterial analysis by observing effect on chemical indicatorSodium azideStabilizing Agents
The invention relates to a reagent and particularly relates to a method for preparing a stable micro-emulsion kit. The kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises a TAPS buffered solution, Colipase, sodium deoxycholate, sodium chloride, calcium chloride, taurodeoxycholic acid and sodium azide; and the pH value of the reagent R1 is 8.0. The reagent R2 comprises a tartaric acid buffered solution, sodium cholate, taurodeoxycholic acid, a preservative, sodium chloride and a stabilizing agent; and the pH value of the reagent R2 is 3.5. Compared with the prior art, the improved emulsifying method can be used for solving the problems of poor stability and large batch difference of the reagent on the basis that the reagent does not influence other performances.
Owner:AILEX TECH GRP CO LTD +1
Feed for preparation of alcoholic liver animal model
The invention belongs to the technical field of medical animal model feed and discloses feed for preparation of an alcoholic liver animal model. The feed for mice is prepared from casein, L-cystine, DL-methionine, maltodextrin, meal cellulose, xanthan gum, choline bitartrate, a mineral premix, a vitamin premix, cholesterol, sodium cholate, fish oil, alcohol and water. The feed for preparation of an alcoholic liver animal model can gradually cause mouse alcoholic fatty liver, hepatitis and liver fibrosis. The model has features similar to human diseases and pathology has a certain development process. A modeling method is simple and easy and has a high success rate, a low death rate, good repeatability and a wide application prospect.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Itraconazole liposome and preparation process thereof
InactiveCN1969830AHighly toxicStrong side effectsOrganic active ingredientsAntimycoticsMANNITOL/SORBITOLPhosphate
The invention discloses an eosin liposome and making method, which comprises the following parts: 18-22mg eosin, 505-509mg lecithin, 90-95mg cholesterol, 73-77mg sodium deoxycholate, 505-509mg mannitol, 505-509mg lactose and 18-22mL phosphate buffer with pH value at 5.5-6.5. the making method comprises the following steps: (1) adding eosin, sodium deoxycholate, lecithin and cholesterol into chloroform; (2) decompressing; evaporating chloroform; (3) adding lactose and mannitol into phosphate buffer; (4) emulsifying phosphate buffer without fat film; (5) freezing; drying to obtain the product.
Owner:HARBIN MEDICAL UNIVERSITY
Method for preparing hexagonal boron nitride nanometer layer sheet
InactiveCN106744738AAchieve solid phase exfoliationReduce usageNitrogen compoundsNanotechnologyHexagonal boron nitrideRoom temperature
The invention provides a method for preparing a hexagonal boron nitride nanometer layer sheet. The method comprises the following steps of uniformly mixing hexagonal boron nitride raw materials and exfoliant to obtain a mixture, wherein the exfoliant is selected from sodium cholate and sodium dodecyl benzene sulfonate; under the room temperature condition, the mixture is ground to obtain the hexagonal boron nitride nanometer layer sheet. The method has the advantages that the use of a solvent is avoided; the prepared hexagonal boron nitride nanometer layer sheet can be stored for a long time in a solid-state form; the layer sheet gathering and sedimentation process often occurring in the liquid phase is avoided; the effect of taking upon use is achieved.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Composite mesenchymal stem cell functional collagen scaffold and applications thereof
The invention discloses a composite mesenchymal stem cell functional collagen scaffold and applications thereof. According to the present invention, isolated animal fascia is soaked sequentially withacetone, a sodium deoxycholate solution, a Triton X-100 solution, a pepsin aqueous solution and a papain solution, and free-drying is performed to obtain the composite mesenchymal stem cell functionalcollagen scaffold; the composite mesenchymal stem cell functional collagen scaffold is used for acute spinal cord injury and old spinal cord injury, and the comprehensive evaluation results from various aspects show that the composite mesenchymal stem cell functional collagen scaffold can substantially promote the movement function recovery, nerve regeneration, axon myelination and synapse formation of acute and old spinal cord injury dogs; and the functional biomaterial can provide great significance in the repair of spinal cord injury, and can provide theoretical support for the applicationof functional biomaterials in the future.
Owner:BEIJING ZKZKTECH CO LTD
Mouth spray for preventing and treating nausea and emesis after tumor chemotherapy and radiotheraphy and preparation method thereof
InactiveCN101385712AEasy to increase or stop doseReduce efficacyAerosol deliveryPharmaceutical non-active ingredientsDiseaseAdditive ingredient
The invention relates to an oral spray for controlling nausea and vomit of chemotherapy and radiation therapy, the formula of the oral spray is composed of ingredients with the following parts by weight: 5-50 parts of drug absorption enhancer, 2-20 parts of drug active ingredient and 30-90 parts of buffer, the drug absorption enhancer can be any one or the combination of more of the following ingredients: azone, propylene glycol, polysorbate (Tween), ethylene glycol deoxycholic acid sodium salt, brij, sodium decanoate, lauric acid, stearic acid, sodium lauryl sulphate, stearyl alcohol sodium sulfate, dioctyl succinate sodium sulfonate, oleic acid, GK2, menthol and borneol; the drug active ingredient can be any one combination of the following ingredients: palonosetron hydrochloride, granisetron, ondansetron, azasetron and tropisetron; and the ingredients of the buffer are sodium citrate buffer solution and phosphate buffer solution. The oral spray provides a formulation which is safer, painless and convenient for patients with advanced tumor, elderly and weak patients, children patients and the patients who suffer from the metal illness and do not obey the oral administration or the injection drug administration.
Owner:陆飚 +1
Method for preparing astragalus Sanxian soup flexible nano-liposome
InactiveCN104188908AUniform particle sizeHigh encapsulation efficiencyOrganic active ingredientsSkeletal disorderCholic acidAstragaloside
The invention discloses a method for preparing astragalus Sanxian soup flexible nano-liposome. The preparation method comprises the following steps: (1) weighing lecithin, cholesterol, astragaloside, icariin and notoginsenoside R1 according to a ratio, adding the raw materials into a container, and adding a methanol-chloroform mixed solvent, so that the raw materials are fully dissolved; (2) arranging the dissolved raw materials on a rotary evaporator, performing reduced pressure spin evaporation to remove an organic solvent in a constant temperature water bath, and performing vacuum drying overnight; (3) preparing a sodium cholate PBS solution, adding ethylene diamine tetramethylidene phosphoric acid into the sodium cholate PBS solution, adding the mixed solution into the dried raw materials, and performing normal pressure spin evaporation in the constant temperature water bath, thereby preparing primary suspension of the liposome; and (4) ultrasonically oscillating the primary suspension of the liposome, and finally sequentially squeezing the primary suspension to pass through microfiltration membranes with the pore diameters of 0.80mu m, 0.45mu m and 0.22mu m to obtain the flexible nano-liposome. The liposome disclosed by the invention has the advantages of uniform particle size, high encapsulation efficiency and high targeting property.
Owner:GUANGDONG MEDICAL UNIV
Biodegradable magnesium alloy bile duct litholysis knitted bracket and preparation method thereof
InactiveCN102727331APrevents stenosis from closingGuaranteed unobstructedStentsSurgeryDisodium EdetateBiocompatibility Testing
The invention belongs to the field of biomedicine, high molecular materials and magnesium alloy materials, and relates to a biodegradable magnesium alloy bile duct litholysis knitted bracket and a preparation method thereof. A knitted bracket is manufactured by knitting magnesium or magnesium alloy into a porous net structured or spiral tubular structured duct; and sodium cholate or / and edetate disodium containing biodegradable polymer layers is / are coated on the inner and outer surfaces of the knitted bracket to obtain the biodegradable magnesium alloy bile duct litholysis knitted bracket. Clinical using results indicate that the bracket provided by the invention is good in radial support property, capable of keeping bile duct unobstructed, capable of being degraded in a certain time to disappear and free of taking out once again; the biocompatibility is good and nearly no inflammation is generated due to in-vivo activities; and at the same time, through the sodium cholate or / and edetate disodium released by the medicine containing polymer coatings, formed stones can be dissolved and prevented from reforming.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Genetic engineering bacteria generating bile salt hydrolase as well as construction method and application thereof
ActiveCN102220276ALower serum cholesterolIncrease enzyme activityBacteriaHydrolasesGenetic engineeringRecombinant escherichia coli
The invention discloses genetic engineering bacteria generating bile salt hydrolase as well as construction method and application thereof and belongs to the technical field of genetic engineering. According to the invention, the bile salt hydrolase (bsh) of Lactobacillus plantarum BBE7 is connected to an escherichia coli expression vector pET28a(+) through gene cloning by utilizing a recombinant DNA (deoxyribonucleic acid) technology, and Escherichia coli BL21(DE3) is transformed, and a strain of recombinant Escherichia coli BL21 (DE3)-pET28a(+)-bsh capable of generating bile salt hydrolase with relatively high activity is obtained by virtue of screening and identification, wherein the collection number is CCTCC No.M2011115. The total enzymatic activity of the bile salt hydrolase expressed by the strain against glycodeoxycholic acid hydrate (GDCA) is 3.7819 U / ml which is nearly 11 times higher than that of wild bacteria. The method lays a good foundation for the large-scale production of bile salt hydrolase and the reduction of serum cholesterol when being used as functional food.
Owner:JIANGNAN UNIV
Preparation method of flexible liposomes
InactiveCN107669497AImprove deformation abilityImprove the wrapping effectCosmetic preparationsToilet preparationsCholesterolPolyethylene glycol
The invention relates to a preparation method of flexible liposome, which comprises the following preparation steps: taking the following raw materials for use: lecithin, Beijing Shuangxuan Microbial Culture Medium Products Factory; cholesterol, Shanghai Sinopharm Group; sodium cholate, Aladdin Reagent Company Vitamin E, Aladdin Reagent Company; Polyethylene glycol, Tianjin Fuyu Fine Chemical Co., Ltd.; The preparation method of flexible liposome of the present invention, by improving the deformability of liposome, thereby improves coating effect, It has good yield and good practicability.
Owner:SHAANXI EYOUNG TECH
Nystatin flexible liposome as well as gel and preparation method of nystatin flexible liposome
InactiveCN102600079AImprove flexibilityImprove transmittanceOrganic active ingredientsAntimycoticsNystatinumPharmaceutical drug
The invention provides a nystatin flexible liposome and external preparation gel and simultaneously discloses a preparation method of the nystatin flexible liposome. Through the nanometer liposome technology, nystatin is covered and sealed in the liposome, the solubility of the nystatin is enhanced, the liposome recipe is improved, sodium cholate or sodium deoxycholate is added, the flexible liposome is prepared, the liposome pereutaoeous permeation is enhanced, in addition, the nystatin stability can be improved, a nystatin cutaneous penetration path is developed, and the treatment effect is realized in aspects of skin superficial layer or deep infection and systemic fungal infection. The nystatin flexible liposome is prepared into the gel, the detention time of the nystatin flexible liposome on the skin is prolonged, the use is simple and convenient, the cost is low, in addition, the compliance of users is improved, the toxicity is reduced, medicine storage bases can be formed on the skin, medicine is continuously released, and the medicine acting time is prolonged.
Owner:JILIN UNIV
Method for preparing nanocapsule and nanocapsule composite microsphere
InactiveCN101401792AUniform particle sizeUniform sizePowder deliveryOrganic active ingredientsUltrasonic emulsificationMicrosphere
The invention discloses a method for preparing a nanometer capsule and a method for preparing a nanometer capsule composite microsphere. The method for preparing the nanometer capsule comprises the following: step 1, preparing a methotrexate alkaline solution and a PLGA-CH2Cl2 solution; step 2, injecting the methotrexate alkaline solution into the PLGA-CH2Cl2 solution to obtain a W / O colostrum after ultrasonic emulsification; step 3, adding the colostrum into sodium cholate to obtain a W / O / W multiple emulsion after the ultrasonic emulsification; and step 4, placing the obtained multiple emulsion in the sodium cholate to be evaporated through decompression to remove CH2Cl2 so as to obtain a nanometer capsule particle suspension. The method for preparing the nanometer capsule composite microsphere comprises the following: step a, preparing a nanometer capsule; and step b, preparing the nanometer capsule composite microsphere. The nanometer capsule particle prepared by the invention has the characteristics of even size, higher drug loading and encapsulation efficiency, peak value drug release, and smooth drug release.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Smoke filter liquid for cigarette filters as well as preparation method and application of smoke filter liquid
ActiveCN105852208AIncrease moisture contentImprove comfortTobacco smoke filtersActive agentEngineering
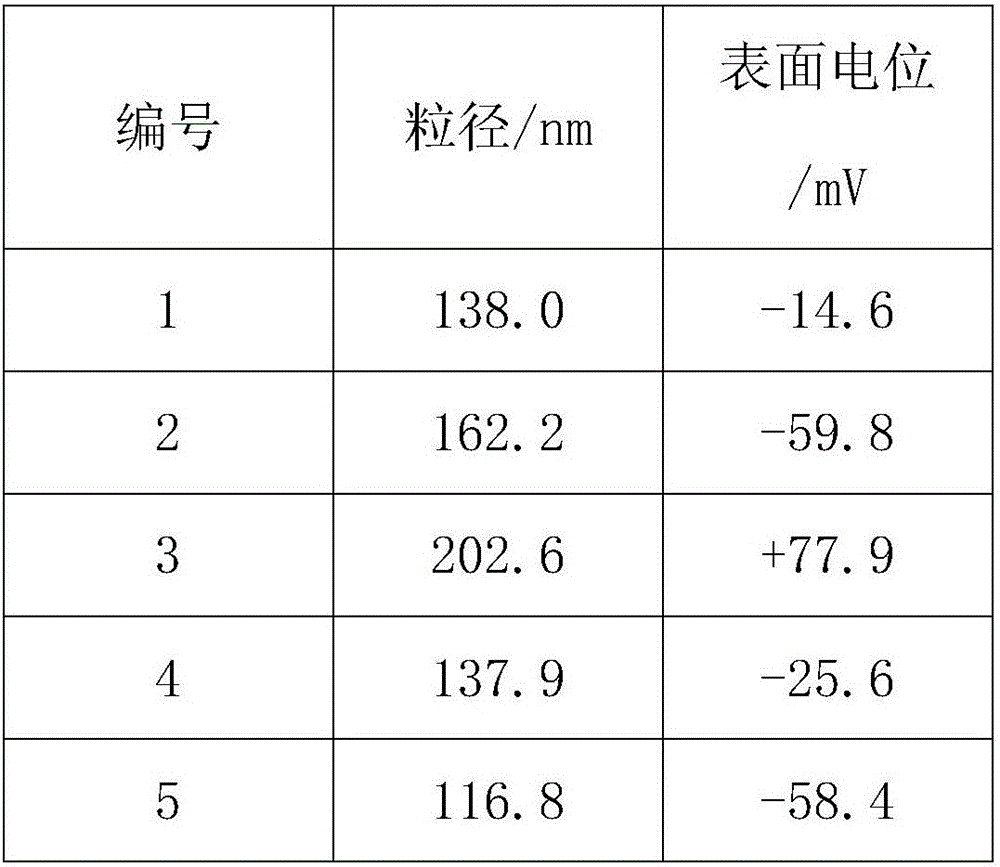
The invention provides smoke filter liquid for cigarette filters as well as a preparation method and application of the smoke filter liquid. The smoke filter liquid is polylactic acid nanometer suspension liquid prepared from an organic phase and a water phase by virtue of a self-emulsifying solvent evaporation method, wherein the organic phase is prepared by dissolving polylactic acid powder into a mixed solvent of acetone and ethanol, the water phase is prepared by dissolving a surfactant into ultrapure water, the particle diameters of polylactic acid nano-particles are 110nm-210nm, and the surface potential is (-65mV)-(+80mV); the mass ratio of the organic phase and the water phase is (1 to 5)-(2 to 5), and the surfactant is one of poloxamer188, lauryl sodium sulfate, cetyl trimethyl ammonium bromide, taurine and sodium cholate. According to the smoke filter liquid, the problems that tar in smoke cannot be well filtered by virtue of existing cigarette filters, and a smoking device for shredded tobacco for water pipes is complex are simultaneously solved, furthermore, the whole preparation process is simple and convenient, and the taste of cigarettes is not influenced.
Owner:HUBEI CHINA TOBACCO IND
Production process of sodium cholate
The invention provides a production process of sodium cholate, and the preparation method comprises the following steps: 1) collecting fresh gallbladder, cutting open the gallbladder skin, collecting the gall, stirring uniformly, adding sodium hydroxide and heating for melting; 2) cooling, siphoning, filtering, stirring and cooling again and precipitating; 3) adding acid and filtering, stirring and precipitating; 4) preparing granular cholic acid, washing, hanging to dryness, and drying to obtain a semi-finished product of sodium cholate; 5) guiding a mixture of alcohol and hydrogen peroxide into the semi-finished product of sodium cholate, stirring uniformly into a feed form, and spinning to dryness to obtain the sodium cholate. By adopting the production process provided by the invention, the production efficiency and the product quality are improved, and the production processes are reduced.
Owner:石汉生
Celastrol flexible liposome, gel and preparation method thereof
PendingCN107149593AOperational securityIdeal particle sizeOrganic active ingredientsAerosol deliveryYolkMedicine
The invention belongs to the field of pharmaceutical preparations and discloses celastrol flexible liposome which is composed of 1 part of celastrol, 1-4 parts of sodium cholate, 2-10 parts of cholesterol and 15-40 parts of egg yolk lecithin. The invention further provides a preparation method of celastrol flexible liposome, compound celastrol flexible liposome gel and a preparation method thereof. The celastrol flexible liposome obtained by the preparation method is ideal in production process operation, safety, particle size and encapsulation rate and high in physical stability; an obtained celastrol flexible liposome suspension can be directly used for preparing the compound celastrol flexible liposome gel without going through steps of separation and purification. In addition, the compound celastrol flexible liposome gel obtained by the preparation method has good heal promoting effect on rat scald wounds.
Owner:THE 118 CLINIC DIV OF THE NO 113 HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY
Vibrio alginolyticus selectivity differential medium
InactiveCN102146429AImprove detection efficiencyOvercome the disadvantages of poor selectivityMicrobiological testing/measurementAgainst vector-borne diseasesBromothymol blueThymol blue
The invention discloses a selectivity differential medium-vibrio alginolyticus selectivity differential medium (VaDM) which uses rapid screened vibrio alginolyticus as a unique target bacterium. The vibrio alginolyticus selectivity differential medium is prepared from leucine, yeast leaching powder, sodium chloride, sodium thiosulfate, sodium cholate, bile powder, ferric citrate, calcium chloride, agar, bromothymol blue, thymol blue and distilled water. In the invention, all components of the VaDM are optimized and combined according to a special leucine aminoase system of vibrio alginolyticus, basic and special nutrition requirement, halophilism and resistance on some bacteriostats, favorable growth of the bacterium is guaranteed, the growth of a plurality of other miscellaneous bacteriacan be effectively inhibited, the target bacterium presents special bacterial colony color and achieves good selecting and distinguishing effects with other bacteria. The invention verifies that VaDMhas an ideal effect on the rapid screening of vibrio alginolyticus by testing bacterium yield, accuracy, precision and sensitivity and comparing the conventional detecting method for detecting a practical sample.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Composition for improving yield of Eimeria maxima oocysts, and preparation method thereof
ActiveCN107019111AIncrease productionScientific and reasonable compositionAnimal feeding stuffAccessory food factorsEpitheliumBerberine
The present invention relates to a composition for improving the yield of Eimeria maxima oocysts, and a preparation method thereof, wherein the composition comprises, by weight, 10-30 parts of pulsatilla chinensis, 1-10 parts of vitamin A, 0.5-10 parts of vitamin E, 2-30 parts of berberine, 0.5-5 parts of sodium cholate, and 10-90 parts of stone powder. According to the present invention, the composition has characteristics of scientific and reasonable formula and simple process, can improve the yield of Eimeria maxima oocysts by maintaining the integrity of the intestinal tract epithelium mucous membrane, reducing the damage of intestinal tract gram-negative bacteria on the intestinal tract epithelial cells, improving the excystation rate of sporozoites, and other effects, and can improve the production efficiency of the Eimeria maxima vaccine, reduce the production cost, and save the social resources.
Owner:TIANJIN HLINTE BIOTECH CO LTD
Liposome ointment containing heparin kind medicine and its preparation method
InactiveCN1478485AEnhanced inhibitory effectOrganic active ingredientsAerosol deliveryThrombusCold injury
A lipid ointment for treating thrombotic diseases, cold injury, burn, superficial phlebitis and eczema contains heparin medicines, auxiliary, phosphatide, cholesterin, sodium cholate and hydrophilic high-molecular polymer. Its preparing process is also disclosed.
Owner:SHANDONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com