Celastrol flexible liposome, gel and preparation method thereof
A technology of tripteryglide and flexible liposomes, which is applied in liposome delivery, liquid delivery, pharmaceutical formulations, etc., can solve the problem of strong irritation of excipients, complicated preparation process, and encapsulation efficiency of tripteryglide liposomes. Unsatisfactory and other problems, to achieve the ideal effect of promoting healing, good effect, particle size and encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The main experimental instruments and reagents are as follows:
[0041] High performance liquid chromatography: Agilent Technologies Co., Ltd.
[0042] Ultrasonic cell disruptor: Scientz-750F, Ningbo Xinzhi Biotechnology Co., Ltd.
[0043] Laser particle size analyzer: Nano-ZS, Malvern Instruments Ltd., UK
[0044] Transdermal Absorption Apparatus: HC-188, Tianjin Zhengtong Technology Co., Ltd.
[0045] Ready-to-use dialysis bags (10K MWCO): ThermoFisher Scientific Inc.
[0046] High-purity egg yolk lecithin (PC-98T): Shanghai Evert Pharmaceutical Technology Co., Ltd.
[0047] High-purity cholesterol: Shanghai Everett Pharmaceutical Technology Co., Ltd.
[0048] Sodium cholate: Sinopharm Chemical Reagent Co., Ltd.
[0049] Chloroform, methanol, ethanol: Sinopharm Chemical Reagent Co., Ltd.
[0050] Tween 80: Sinopharm Chemical Reagent Co., Ltd.
[0051] Carbomer: The Lubrizol Corporation of America
[0052] Tripterygium: Aktin Chemicals, Inc.
[0053] Nano silv...
Embodiment 2
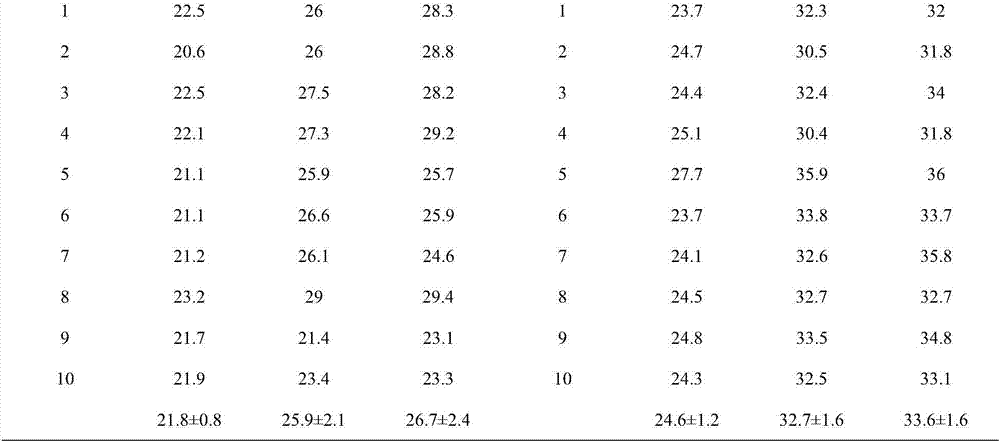
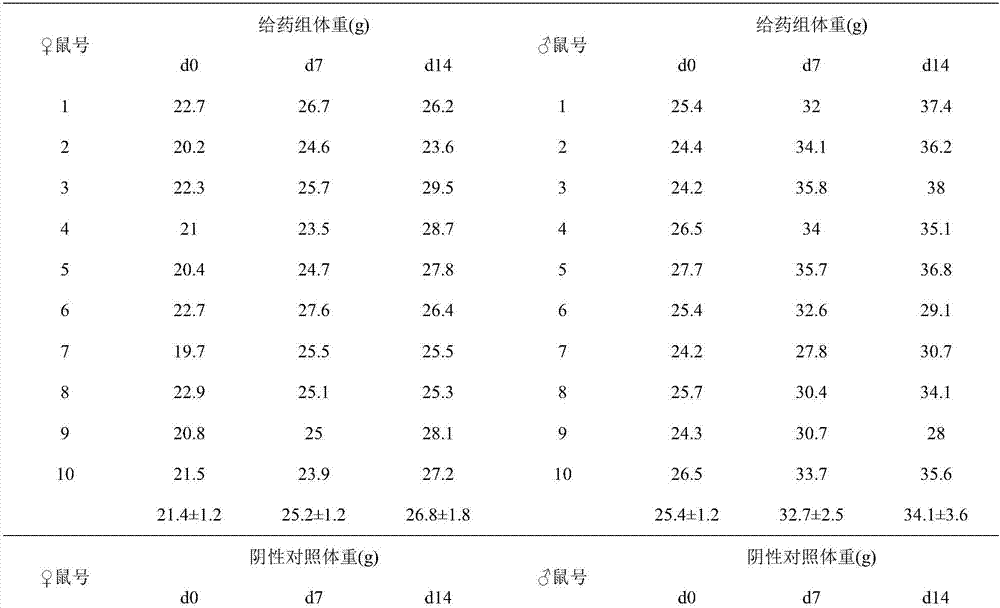
[0059] Precisely weigh 0.2g egg yolk lecithin, 0.05g cholesterol and 0.01g tripterine into a pear-shaped bottle, add 2ml ethanol, fully dissolve and shake well, remove the organic solvent by rotary evaporation at 40°C and 10rpm, add 5ml Sodium cholate solution (3mg / ml), fully hydrated, and ultrasonicated 10 times in an ice bath (120w, working for 2s, with an interval of 2s), to obtain 5ml of tripterine flexible liposome suspension. The average particle diameter of the obtained tripterylide flexible liposome is 110.2nm, the PDI is 0.226, the Zeta potential is -32.6mV, the encapsulation efficiency is 99.7%, and the drug loading capacity of the liposome is 4.2%. After being placed in a refrigerator at 4°C for 2 weeks, there was no significant change in the particle size and encapsulation efficiency, indicating that the liposome has good physical stability.
[0060] Accurately weigh carbomer solid 0.1g, join in 4.495ml nano-silver solution (100 μ g / ml), stir and dissolve, add 0.40...
Embodiment 3
[0062] Accurately weigh 0.2g egg yolk lecithin, 0.05g cholesterol and 0.01g tripterine into a pear-shaped bottle, add 2ml chloroform, fully dissolve and shake well, remove the organic solvent by rotary evaporation at 40°C and 10rpm, add 5ml Sodium cholate solution (2mg / ml), fully hydrated, and ultrasonicated 10 times in an ice bath (280w, working for 2s, with an interval of 2s), to obtain 5ml of tripterine flexible liposome suspension. The average particle diameter is 114.8nm, the PDI is 0.230, the Zeta potential is -33.1mV, the encapsulation efficiency is 99.8%, and the drug loading capacity of the liposome is 4.3%. After being placed in a refrigerator at 4°C for 2 weeks, there was no significant change in the particle size and encapsulation efficiency, indicating that the liposome has good physical stability.
[0063] Accurately weigh carbomer solid 0.025g, join in 4.57ml nano-silver solution (120 μ g / ml), stir and dissolve, add 0.405ml pure water, 5ml tripterine liposome su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com