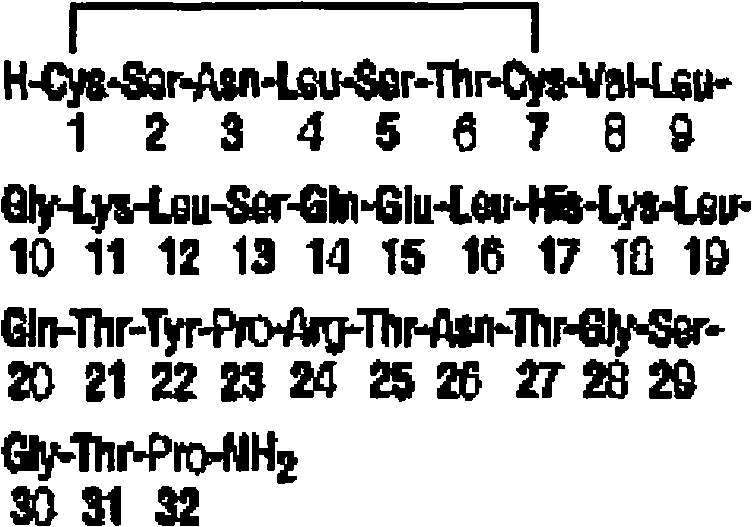Oral solid lipid nano-particle preparation of calcitonin and preparation method thereof
A technology of solid lipid nanometer and lipid nanoparticle is applied in the field of medicines for regulating blood calcium and their preparation, and can solve the problems of non-absorption and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 25
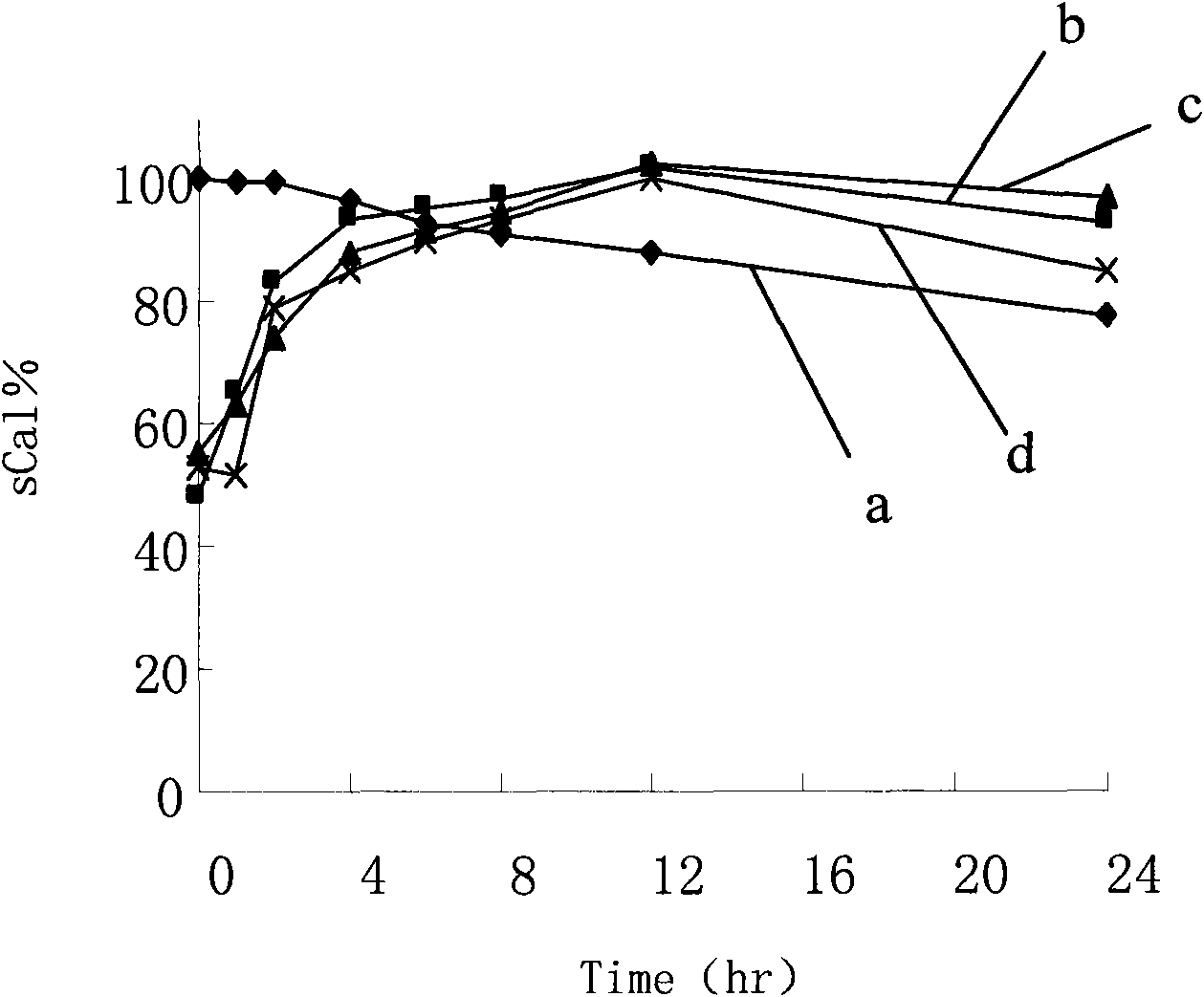
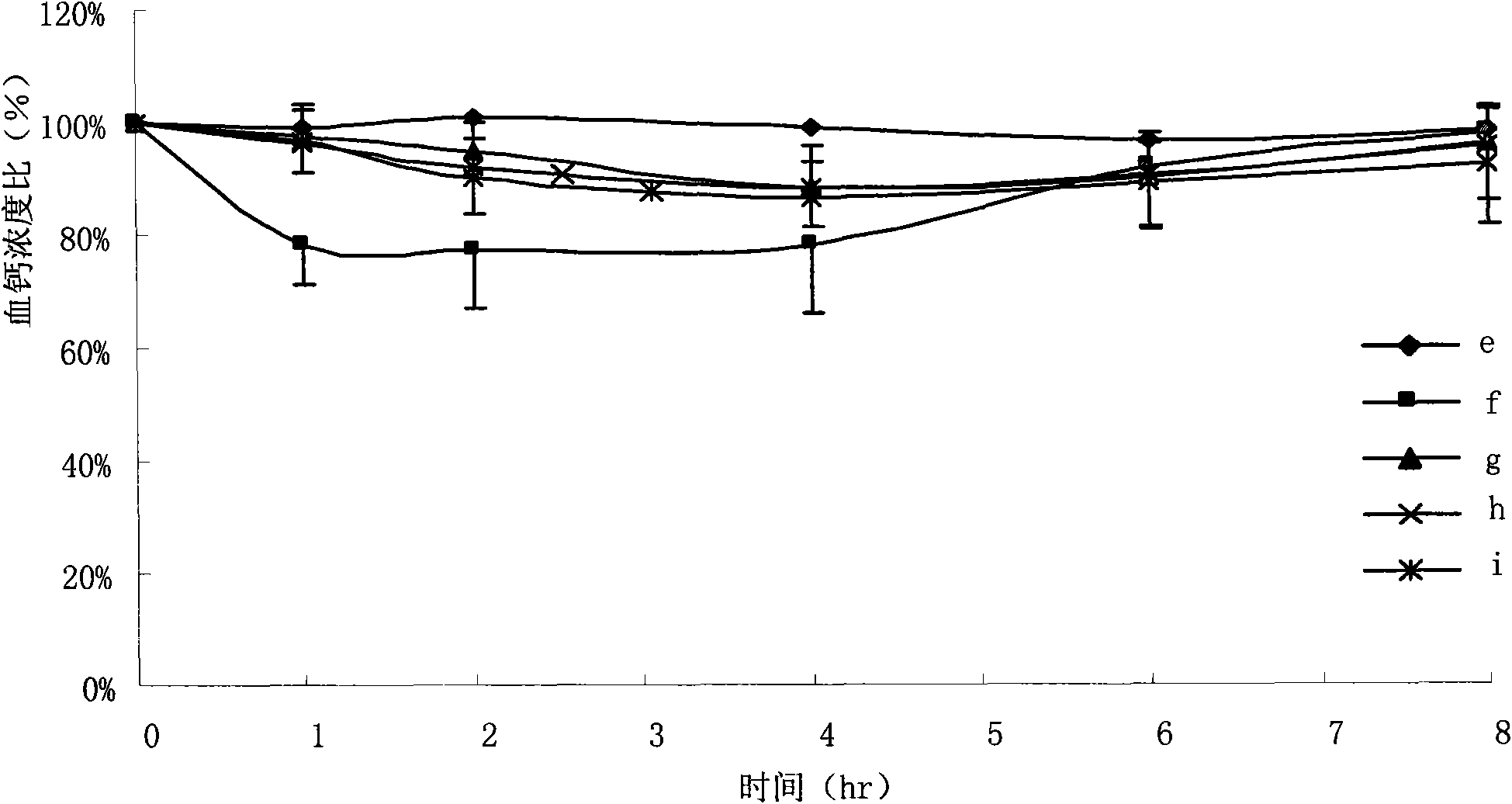
[0080] Take 450 μl of the drug stock solution, 24ml of ethanol, and a total of 400mg of the carrier (monoside ester / stearic acid / oleic acid is 14 / 5 / 1), heat and dissolve at 60°C, pour into the aqueous phase medium (distilled water / 0.01 % sodium cholate), stirring for 5 to 10 minutes. Pre-freeze the nanoparticle suspension in a low-temperature refrigerator at -70°C for 12 hours. After thawing, centrifuge at 25,000×g for 30 minutes, and the precipitate is the collected nanoparticles.
[0081] The determination methods of indirect and direct encapsulation efficiency are the same as in Example 1, except that in the process of direct encapsulation efficiency determination, the melting solvent is PBS solution (pH 1.2) containing 1.0% poloxamer 188. It was found that the direct encapsulation efficiency of the nanoparticle sample was 87.1%.
[0082] Freeze-drying: the nanoparticles obtained by centrifugation were transferred to a freeze-drying container with 0.01% sodium cholate, pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com