Dispersing tablet containing terbinafine hydrochloride
A technology of terbinafine hydrochloride and dispersible tablets, which is applied in the field of medicine, can solve the problems that capsules do not have quick-acting and instant-dissolving properties, and achieve the effects of rapid dissolution, convenient administration, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
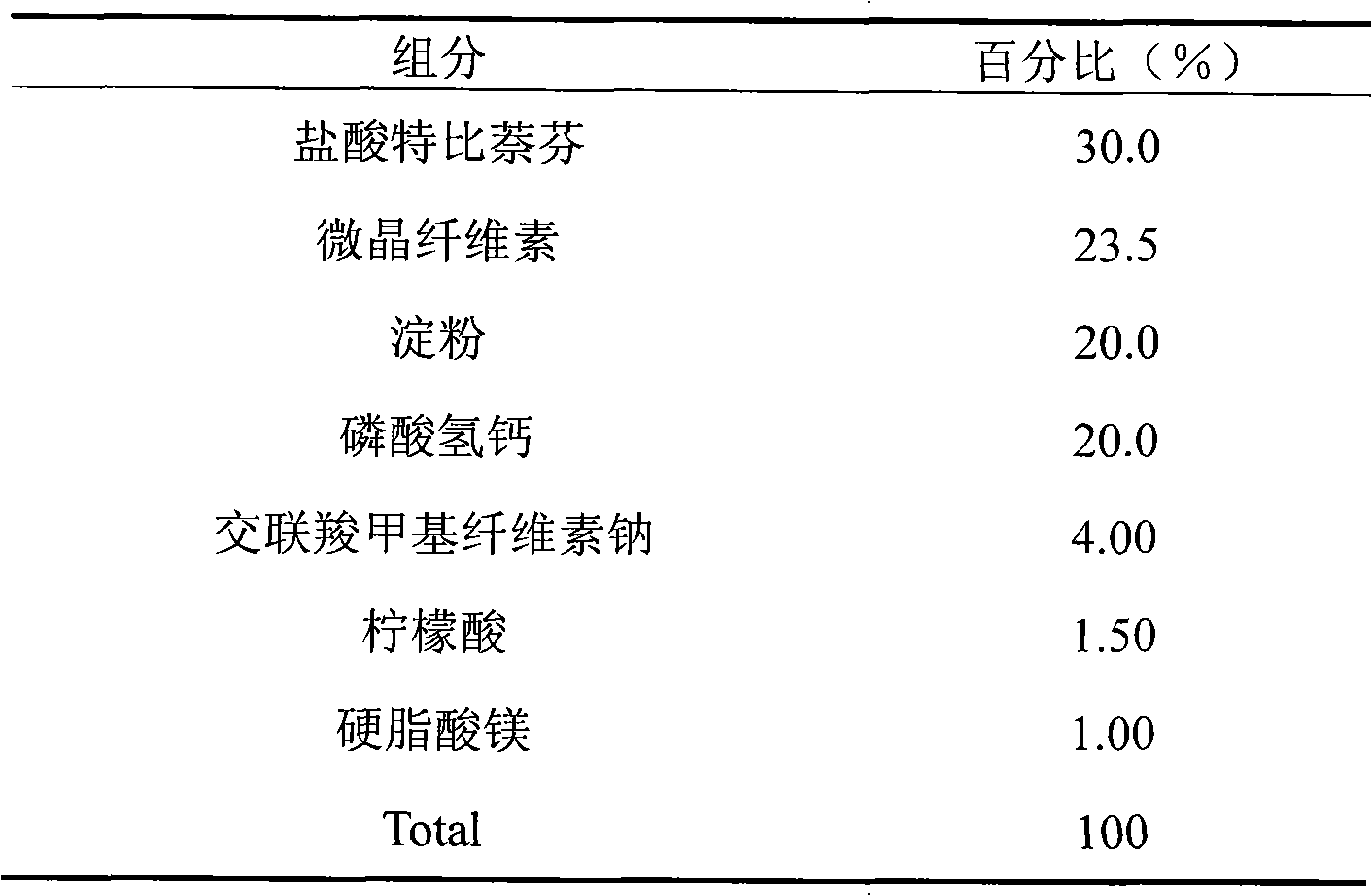
Embodiment 1
[0021]
[0022] Preparation process: Weigh terbinafine hydrochloride, lactose, mannitol, microcrystalline cellulose, and croscarmellose sodium according to the prescription amount, mix well, make soft material, pass through a 20-mesh sieve to granulate, dry, 24 Mesh sieve for granulation; add magnesium stearate and citric acid in prescription ratio, mix evenly, and compress into tablets.
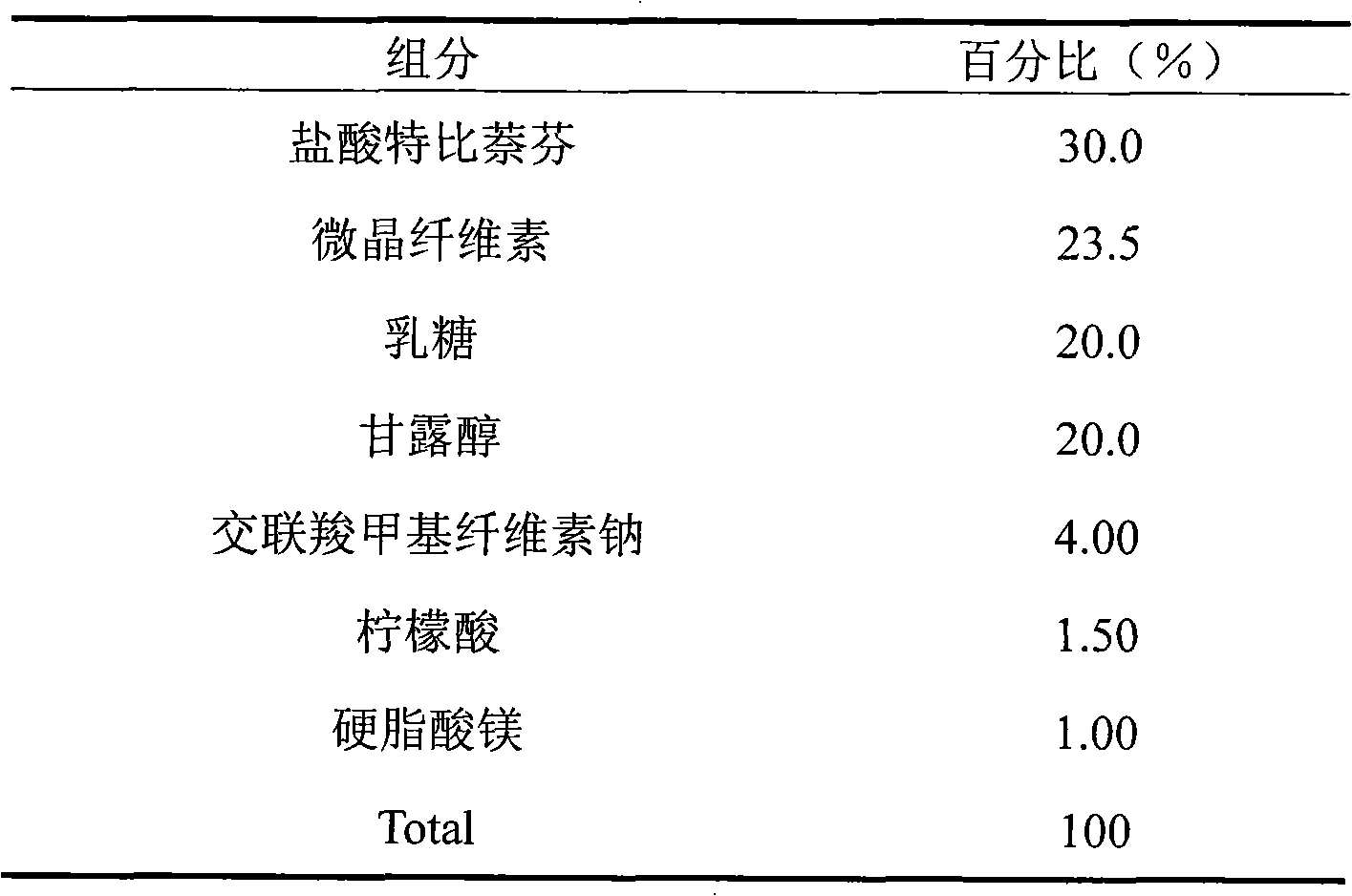
Embodiment 2
[0024]
[0025] Preparation process: Weigh terbinafine hydrochloride, lactose, xylitol, povidone, and sodium carboxymethyl starch according to the prescription quantity, mix well, make soft material, pass through a 20-mesh sieve to granulate, dry, and sieve through a 24-mesh sieve granules; add grape essence and magnesium stearate in the prescribed ratio, mix evenly, and compress into tablets.
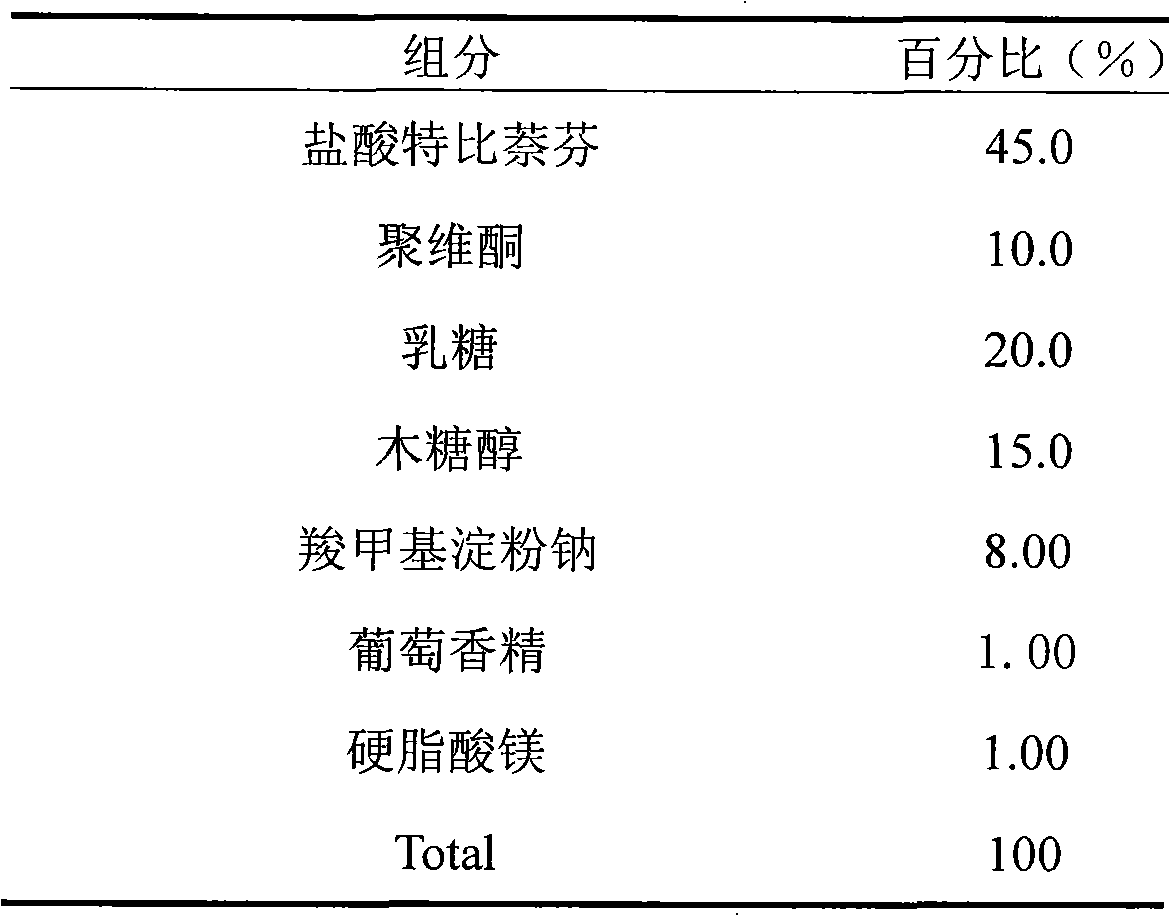
Embodiment 3
[0027]
[0028] Preparation process: Weigh terbinafine hydrochloride, polyethylene glycol, mannitol, low-substituted hydroxypropyl cellulose, and sucrose according to the prescription quantity, mix well, make soft material, granulate through a 20-mesh sieve, dry, 24-mesh Sieve whole grains; add cantaloupe essence and magnesium stearate in the prescribed ratio, mix well, and press into tablets.
[0029] Embodiment 1~3 compares with comparative example 1, commercially available common tablet dissolution rate (n=6, X ± SD)
[0030]
[0031] The results show that: the dissolution rate of the dispersible tablet at each time point is significantly higher than that of the commercially available common tablet, and the dissolution rate of the sample prepared in Example 1 is better than that of the sample of Comparative Example 1 at each time point.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com