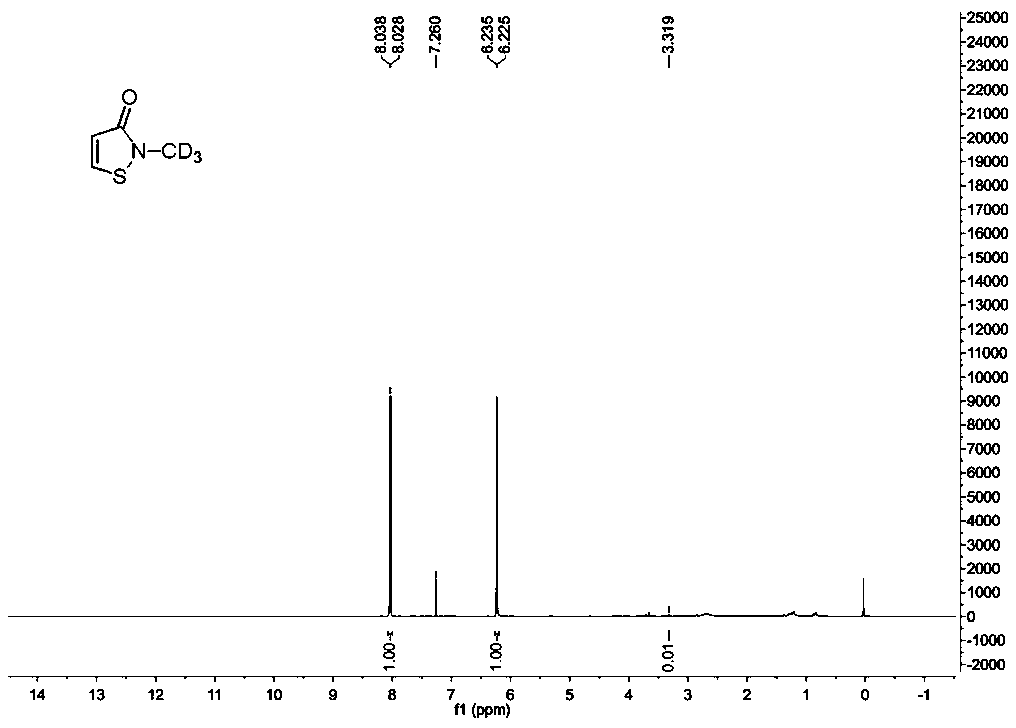
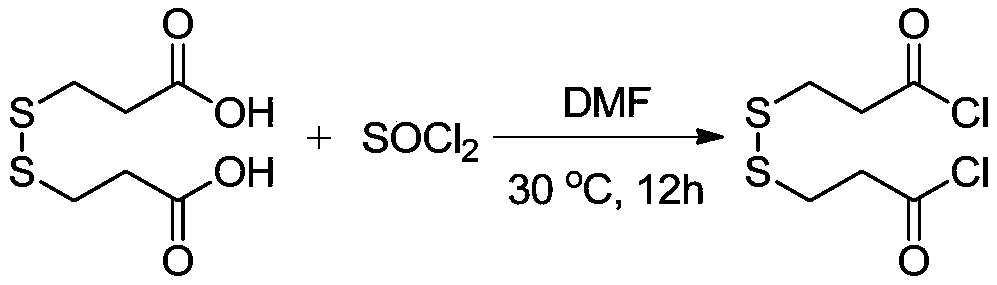
Synthesis method of stable isotope deuterium labeled 2-methyl-4-isothiazole-3-one
A technology of stable isotopes and synthetic methods, applied in the field of isotope deuterium-labeled compound synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A synthetic method for labeling 2-methyl-4-isothiazol-3-one with stable isotope deuterium, comprising the following steps:
[0026] Step 1: Select a 100mL schlenk tube and place it on a flame blower, dry the reaction tube under vacuum conditions, and cool the reaction tube under a nitrogen atmosphere. Then add a stirring bar and 3,3'-dimercaptodipropionic acid (6.3 g, 30 mmol) under a nitrogen atmosphere, draw thionyl chloride (4.4 mL, 60 mmol) into the reaction solution quickly with a 5 ml syringe, In addition, 6 drops (about 0.3 mL) of N,N-dimethylformamide were added dropwise to the reaction solution as a catalyst to speed up the reaction. The reaction tube was placed in an oil bath, the oil bath was stably controlled at 30° C., and vigorously stirred for 12 hours, the reaction solution slowly changed from colorless to yellow. The reaction system was lowered to room temperature, and the volatile matter in the solution was removed in a vacuum environment with an oil ...
Embodiment 2
[0036]A synthetic method for stable isotope deuterium-labeled 2-methyl-4-isothiazol-3-one, step 1 and step 2 are the same as in Example 1, the difference is: step 3: deuterated amide (242mg, 1mmol) Place in a 25mL round-bottomed flask, and add 3mL of dichloromethane solvent into the reaction flask. The round bottom flask was placed in an ice-water bath, and sulfuryl chloride (270mg, 2mmol) was slowly added dropwise into the flask. At this time, the solids in the system dissolved slowly and a relatively uniform suspension appeared. The reaction system was continuously stirred in an ice-water bath for about 1 hour, and then continued to stir at room temperature for about 15 hours. At this time, the reaction system became a white suspension. Add potassium carbonate to the reaction system until there were no bubbles, and continue to stir for 10 minutes. Silica gel (2g, 200-300m) was added to the reaction system, and the volatile matter in the system was removed with a rotary evapo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com