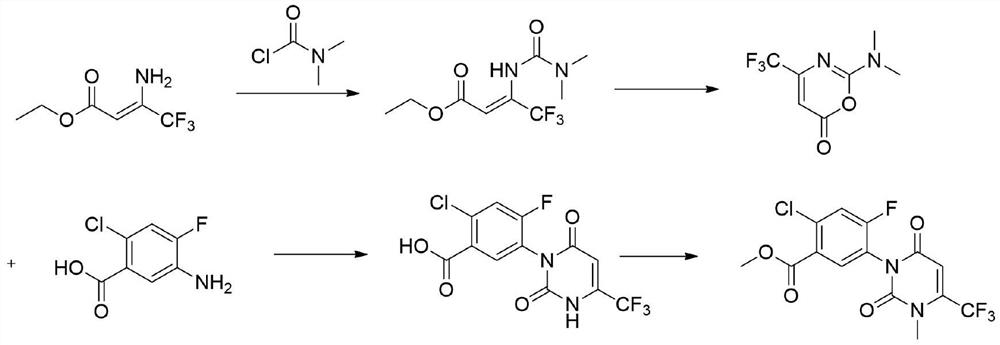
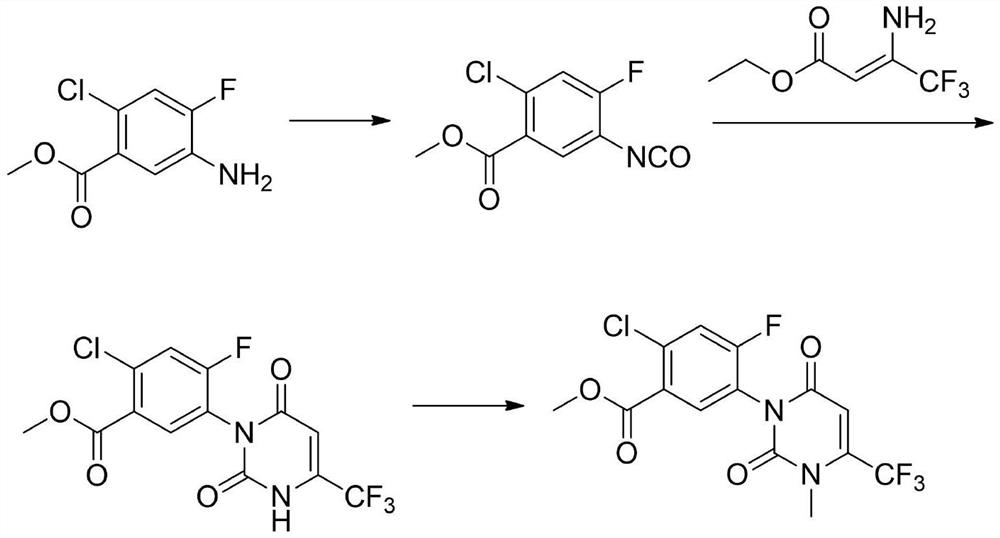
Preparation method of saflufenacil intermediate
A technology for saflufenacil and an intermediate, which is applied in the field of preparation of saflufenacil intermediates, can solve the problems of low yield, highly harmful and toxic reagent raw materials, complicated operation and the like, and achieves simple and good operation. Prospects for industrialization and the effect of less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
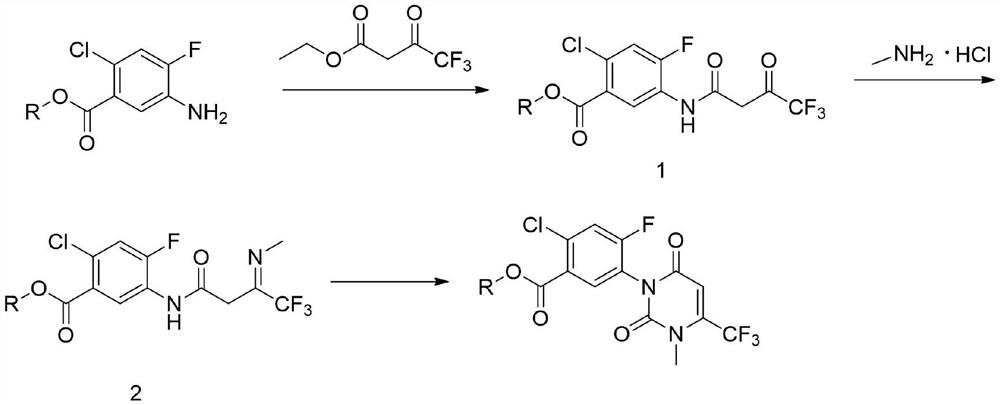
[0034] A preparation method of a saflufenacil intermediate, comprising the steps of:
[0035] a) Preparation of intermediate (1)
[0036] Dissolve 100g of methyl 2-chloro-4-fluoro-5-aminobenzoate and 108g of ethyl trifluoroacetoacetate in 500ml of toluene, then add 9.8g of sodium hydroxide, heat up to 100°C, react for 2h and then cool to room temperature , adding 1 mol / L dilute hydrochloric acid for washing, liquid separation, drying and concentration to obtain 150.1 g of intermediate (1), with a yield of 90%;
[0037] b) Preparation of intermediate (2)
[0038] Dissolve 150.1g of intermediate (1) and 45g of methylamine hydrochloride in 500ml of o-xylene, heat up to 140°C, react for 4 hours, cool to 0°C, and filter after crystallization to obtain 143.6g of intermediate (2) , yield 92%;
[0039] c) 2-chloro-4-fluoro-5-(3-methyl-2,6-dione-4-trifluoromethyl-2,3-dihydropyrimidinyl-1(6H)-yl)benzoic acid Preparation of methyl esters
[0040] Dissolve 143.6g of intermediate (2) ...
Embodiment 2
[0042] A preparation method of a saflufenacil intermediate, comprising the steps of:
[0043] a) Preparation of intermediate (1)
[0044] Dissolve 100g of methyl 2-chloro-4-fluoro-5-aminobenzoate and 134g of ethyl trifluoroacetoacetate in 500ml of chlorobenzene, then add 27g of sodium ethylate, heat up to 120°C for 3 hours, cool to room temperature, add Washing with 1 mol / L dilute hydrochloric acid, separating, drying and concentrating gave 151.9 g of intermediate (1), with a yield of 91%;
[0045] b) Preparation of intermediate (2)
[0046] Dissolve 151.9g of intermediate (1) and 55g of methylamine hydrochloride in 500ml of o-dichlorobenzene, heat up to 160°C for 5 hours, cool to 2°C, and filter after crystallization to obtain 143.9g of intermediate (2 ), yield 90%;
[0047] c) 2-chloro-4-fluoro-5-(3-methyl-2,6-diketone-4-trifluoromethyl-2,3-dihydropyrimidinyl-1(6H)-yl)benzoic acid Preparation of methyl esters
[0048] Dissolve 143.9g of intermediate (2) in 400ml of N,N-di...
Embodiment 3
[0050] A preparation method of a saflufenacil intermediate, comprising the steps of:
[0051] a) Preparation of intermediate (1)
[0052] Dissolve 100g of methyl 2-chloro-4-fluoro-5-aminobenzoate and 178g of ethyl trifluoroacetoacetate in 500ml of toluene, then add 47.6g of triethylamine, heat up to 140°C, react for 4 hours and then cool to room temperature , adding 1mol / L dilute hydrochloric acid for washing, liquid separation, drying and concentration to obtain 153.7g of intermediate (1), with a yield of 92%;
[0053] b) Preparation of intermediate (2)
[0054] 153.7g of intermediate (1) and 60g of methylamine hydrochloride were dissolved in 500ml of o-dichlorobenzene, heated to 180°C for 6 hours, cooled to 5°C, crystallized and filtered to obtain 148.4g of intermediate ( 2), yield 93%;
[0055] c) 2-chloro-4-fluoro-5-(3-methyl-2,6-diketone-4-trifluoromethyl-2,3-dihydropyrimidinyl-1(6H)-yl)benzoic acid Preparation of methyl esters
[0056] Dissolve 148.4g of intermediat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com