Preparation of N-methy-3,5-ditrifluo-aniline
A technology of trifluoromethylbenzylamine and trifluoromethylbenzaldehyde, which is applied in the field of preparing N-methyl-3 by metal catalytic hydrogenation, can solve the problem of low reaction yield, high preparation cost and cyanoborohydrogenation Sodium is expensive and other problems, to achieve the effect of high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
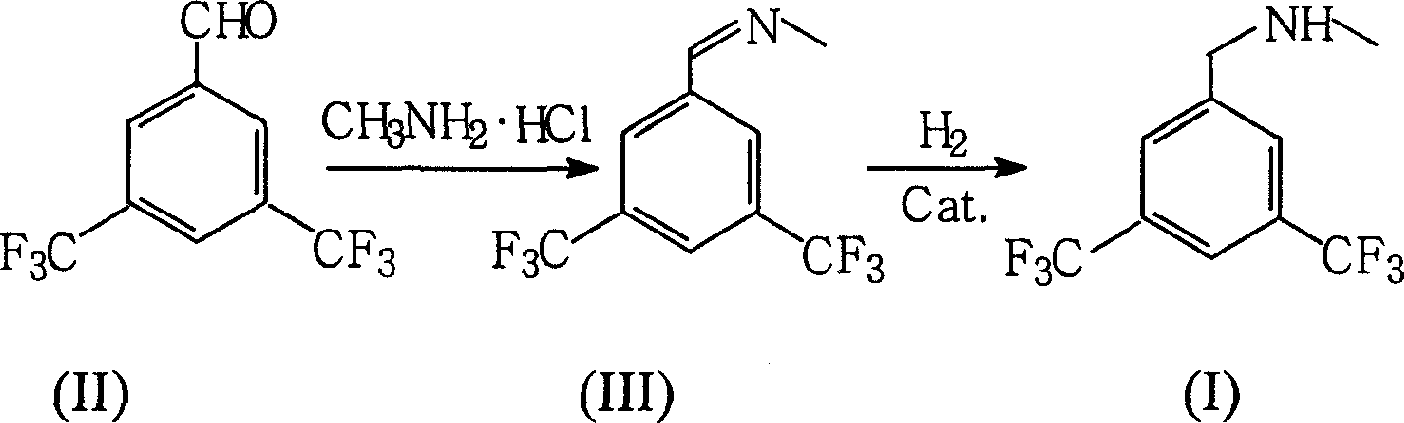
[0010] Embodiment: the preparation of N-methyl-3,5-bistrifluoromethylbenzylamine
[0011] N-methyl-3,5-bistrifluoromethylbenzylamine can be prepared through the following synthetic route.
[0012]
[0013] Add 50g of 3,5-bistrifluoromethylbenzaldehyde (II) and 50g of tetrahydrofuran into a 1000ml four-necked flask equipped with a stirrer, a thermometer, and a low-temperature cold water bath, cool down to 0-5°C, keep this temperature, and Under vigorous stirring, a solution of 50 g of methylamine hydrochloride dissolved in 250 ml of methanol and 75 ml of triethylamine were successively added dropwise, and after the dropwise reaction, the reaction solution was transferred to a 1000 ml autoclave. Under stirring, add R-Ni catalyst 2.5g in autoclave, use N 2 Replace the air in the kettle, heat the temperature inside the kettle to 80°C, and slowly fill the kettle with H 2 , until the kettle pressure was 4MPa, after continuing to react at this temperature for 6h, the catalyst R-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com