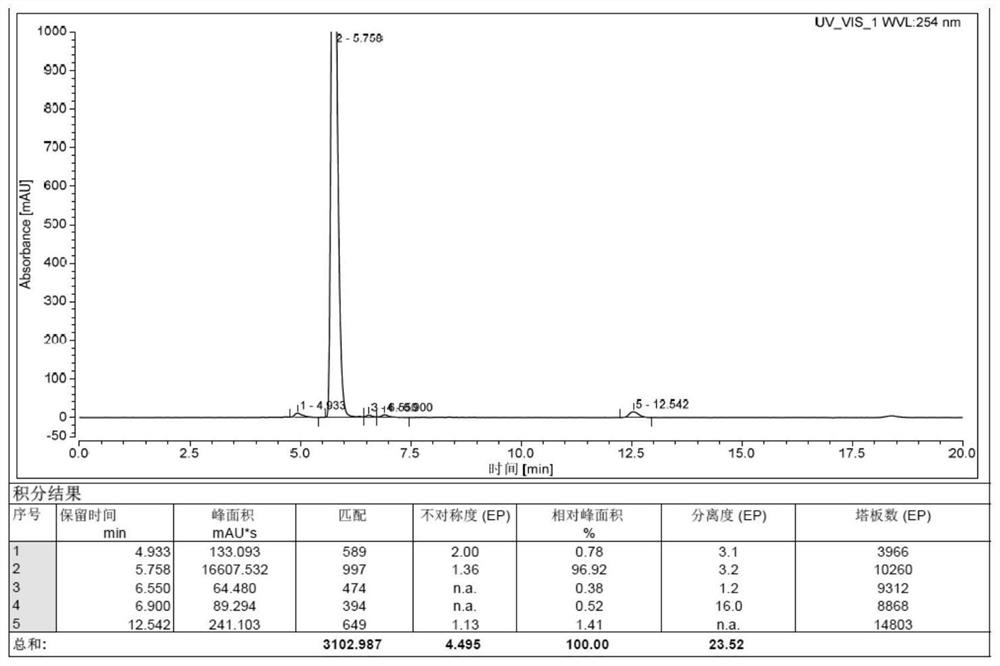
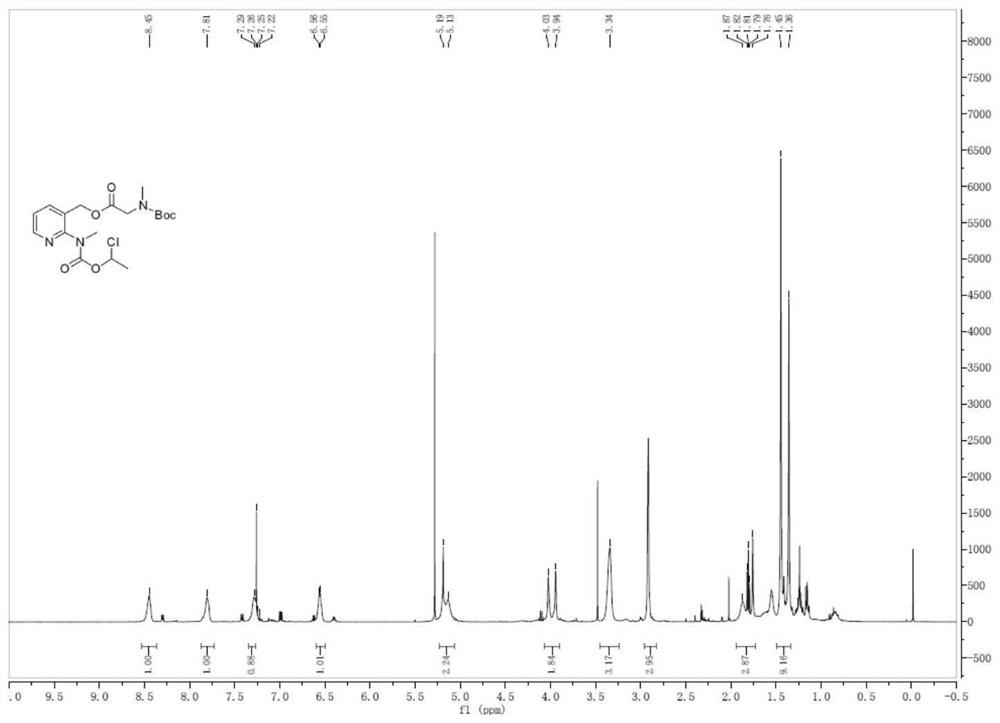
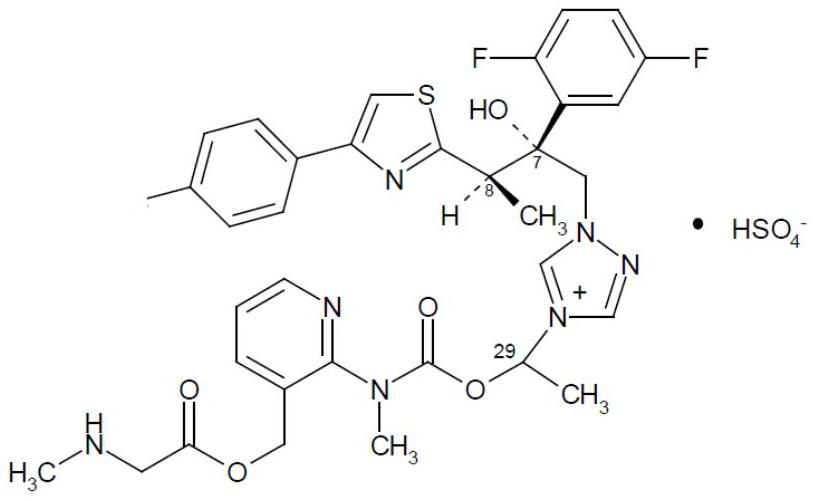
Preparation method of isavuconazonium sulfate intermediate
A technology of isavuconazolium and intermediates, applied in the field of drug synthesis, can solve the problems of high risk of lithium tetrahydrogen, unfavorable industrial production, and inconvenient post-processing, so as to save energy consumption, be beneficial to environmental protection, and reduce side effects. less responsive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation method of isavuconazolium sulfate intermediate of the present invention, the detailed steps of this preparation method are as follows:
[0051] a. Synthesis of 1-chloroethyl methylcarbamate:
[0052] Add 1500 mL of absolute ethanol and 204 g (3 mol) of sodium ethoxide into the reaction flask for stirring and dissolving. After fully dissolving, add 67.5 g (1 mol) of methylamine hydrochloride and stir for 30 min at room temperature. After the reaction, cool the resulting reaction solution to 0 ℃;
[0053] Then 143g (1mol) 1-chloroethyl chloroformate is dissolved in 1500mL dehydrated alcohol, and the gained 1-chloroethyl chloroformate ethanol solution after dissolving is slowly dripped in the above-mentioned cooling gained reaction solution, and the dropping time is 30min; After the dropwise addition, react at 0°C for 2h, filter after the reaction, and the obtained filtrate is methylcarbamate-1-chloroethyl ethanol solution;
[0054] b, (3-hydroxymethyl-py...
Embodiment 2
[0062] The preparation method of isavuconazolium sulfate intermediate of the present invention, the detailed steps of this preparation method are as follows:
[0063] a. Synthesis of 1-chloroethyl methylcarbamate:
[0064] Add 1360mL of absolute ethanol and 136.1g (2mol) of sodium ethoxide into the reaction flask and stir to dissolve. After fully dissolving, add 87.8g (1.3mol) of methylamine hydrochloride and stir at room temperature for 60min. After the reaction, cool the resulting reaction solution to -20°C;
[0065] Then 143g (1mol) 1-chloroethyl chloroformate is dissolved in 2145mL dehydrated alcohol, and the gained 1-chloroethyl chloroformate ethanol solution after dissolving is slowly dripped in the reaction solution obtained by the above-mentioned cooling, and the dropping time is 60min; After the dropwise addition, react at -10°C for 3h, filter after the reaction, and the obtained filtrate is methylcarbamate-1-chloroethyl ethanol solution;
[0066] b, (3-hydroxymethy...
Embodiment 3
[0074] The preparation method of isavuconazolium sulfate intermediate of the present invention, the detailed steps of this preparation method are as follows:
[0075] a. Synthesis of 1-chloroethyl methylcarbamate:
[0076] Add 1910mL of absolute ethanol and 272.2g (4mol) of sodium ethoxide into the reaction flask and stir to dissolve. After fully dissolving, add 81.0g (1.2mol) of methylamine hydrochloride and stir at room temperature for 45min. After the reaction, cool down the resulting reaction solution to -10°C;
[0077] Then 143g (1mol) 1-chloroethyl chloroformate is dissolved in 1700mL dehydrated alcohol, and the gained 1-chloroethyl chloroformate ethanol solution after dissolving is slowly dripped in the reaction solution obtained by the above-mentioned cooling, and the dropping time is 45min; After the dropwise addition, react at -5°C for 3h, filter after the reaction, and the obtained filtrate is methylcarbamate-1-chloroethyl ethanol solution;
[0078] b, (3-hydroxym...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com