Preparation of pyrrole sulfonic acid compound salt type
A technology of pyrrole and inorganic acid salts, which is applied in the field of chemical compounds and can solve problems such as rebound and affecting the therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
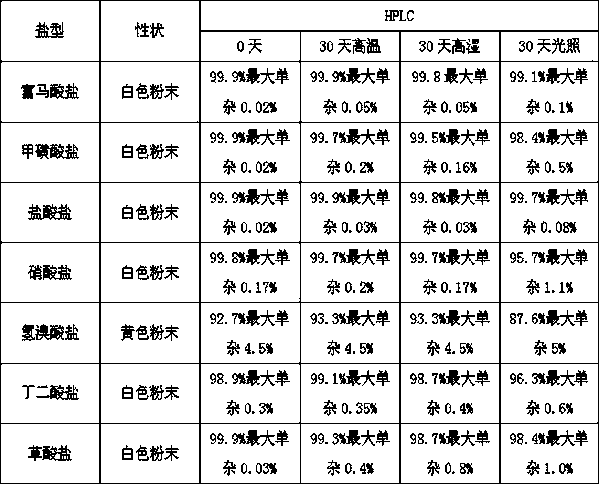
Image
Examples
Embodiment 1
[0025] 1-(5-(2-fluorophenyl)-1-3-(3-methoxypropoxy)benzenesulfonyl chloride)-1H-pyrrol-3-yl)-N-methylamine fumarate
[0026] In a 100 mL one-necked bottle, add 1-(5-(2-fluorophenyl)-1-3-(3-methoxypropoxy)benzenesulfonyl chloride)-1H-pyrrol-3-yl)-N- Methylamine (5.0 g, 11.6 mmol) and ethyl acetate (25 mL) were stirred and dissolved at room temperature (25°C), and fumaric acid (1.6 g, 15.20 mmol) was added to the reaction system, stirred and heated to 50 ℃, and stirred at this temperature for 2h, a solid precipitated out, the solid was filtered out, and washed with ethyl acetate (10 mL*2 times), and the obtained solid was dried in a vacuum oven (35°C) for 4 hours to obtain The white solid was 4.8 g, and the yield was 73%.
[0027] 1 HNMR (DMSO- d 6; 500 MHZ) δ (ppm) 7.6 (s, 1H), 7.48 (m, 2H), 7.28 (m, 1H), 7.20 (m, 2H), 7.09 (m, 2H), 6.83 (s, 1H) , 6.58 (s, 2H), 6.38 (s, 1H), 3.96 (m, 2H), 3.76 (s, 2H), 3.45 (m, 2H), 3.26 (s, 3H), 2.39 (s, 3H), 1.92 (m,2H).
Embodiment 2
[0029] 1-(5-(2-fluorophenyl)-1-3-(3-methoxypropoxy)benzenesulfonyl chloride)-1H-pyrrol-3-yl)-N-methylamine methanesulfonate
[0030] In a 100 mL one-necked bottle, add 1-(5-(2-fluorophenyl)-1-3-(3-methoxypropoxy)benzenesulfonyl chloride)-1H-pyrrol-3-yl)-N- Methylamine (5.0 g, 11.6 mmol) and ethyl acetate (25 mL) were stirred and dissolved at room temperature (25°C), methanesulfonic acid (1.3 g, 13.9 mml) was added dropwise to the reaction system, and at this temperature After stirring for 2 hours, a solid precipitated out, and the solid was filtered out and washed with ethyl acetate (8 mL*2 times), and the obtained solid was dried in a vacuum oven (35°C) for 4 hours to obtain 2.8 g of off-white solid. The rate is 45.9%.
[0031] 1 HNMR (DMSO- d 6; 500 MHZ) δ (ppm) 7.60 (s, 1H), 7.43 (m, 2H), 7.24 (m, 1H), 7.21 (m, 2H), 7.05 (m, 2H), 6.81 (s, 1H) , 6.35 (s, 1H), 3.86 (m, 2H), 3.70 (s, 2H), 3.45 (m, 2H), 3.23 (s, 3H), 2.82 (s, 3H), 2.38 (s, 3H), 1.91 (m,2H).
Embodiment 3
[0033] 1-(5-(2-fluorophenyl)-1-3-(3-methoxypropoxy)benzenesulfonyl chloride)-1H-pyrrol-3-yl)-N-methylamine hydrochloride
[0034] In a 100 mL one-necked bottle, add 1-(5-(2-fluorophenyl)-1-3-(3-methoxypropoxy)benzenesulfonyl chloride)-1H-pyrrol-3-yl)-N- Methylamine (5.0 g, 11.6 mmol) and ethyl acetate (30 mL) were stirred and dissolved at room temperature (25°C), and about 7 ml of ethyl acetate solution (2M) of hydrochloric acid was added dropwise to the reaction system. Stir at high temperature for 2h, no solid precipitates, dry under reduced pressure to remove the solvent to obtain a solid, disperse the resulting object in ethyl acetate (10ml), stir at room temperature for 1h, filter with suction, and wash with ethyl acetate (4ml*2). The obtained solid was dried in a vacuum oven (35°C) for 4 hours to obtain 4.8 g of off-white solid with a yield of 89.1%.
[0035] 1 HNMR (DMSO- d6; 500 MHZ) δ (ppm) 7.63 (s, 1H), 7.58 (m, 2H), 7.38 (m, 1H), 7.32 (m, 2H), 7.19 (m, 2H), 6.93 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com