Preparation process of benzofuran with amide side chain
A preparation process, benzofuran technology, applied in the field of benzofuran preparation process, can solve the problems of difficult separation, high cost, difficult to scale up production of synthesis process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
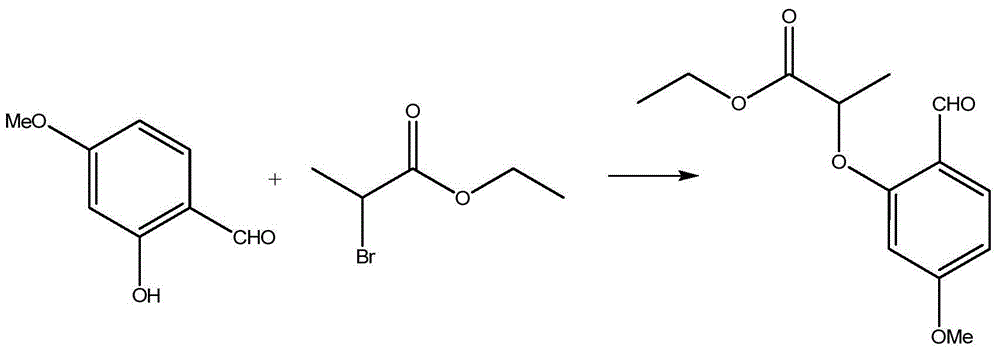
[0014] Embodiment 1: preparation 2-(2-formyl-5-methoxyphenoxy)-propionic acid ethyl ester
[0015]
[0016] Add 92.1 g of 2-hydroxyl-4-methoxybenzaldehyde, 120.7 g of ethyl 2-bromopropionate, 92 g of potassium carbonate and 900 ml of dimethylformamide (DMF, Dimethylformamide) in a 2L reaction flask, and the reaction temperature Raise the temperature to 60°C-90°C, preferably 70°C-90°C, preferably 80°C-90°C, the best implementation temperature is 90°C, and keep at this temperature for 2 hours until the reaction is complete. Then, the reaction intermediate was diluted into 2700ml of water, and the reaction intermediate was extracted three times with 1000ml ethyl acetate, and then the extracts obtained after three extractions with ethyl acetate solution were combined, and then saturated with Wash with brine, and dry with anhydrous sodium sulfate, use distillation to remove the ethyl acetate contained in the reaction intermediate to obtain the first product, 2-(2-formyl-5-methox...
Embodiment 2
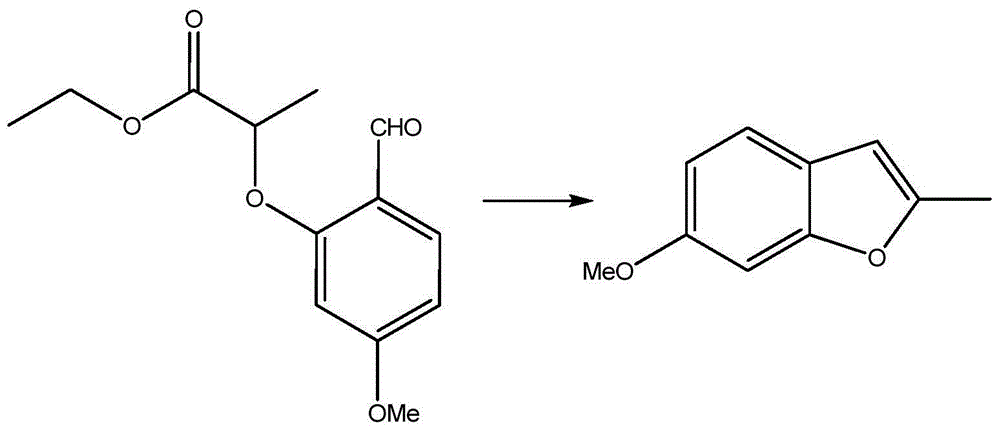
[0017] Embodiment 2: prepare 2-methyl-6-methoxybenzofuran
[0018]
[0019] In a 1L reaction flask, add the first product prepared in Example 1, 100.8 g of ethyl 2-(2-formyl-5-methoxyphenoxy)-propionate and 500 ml of dioxane, and mix them in Stir at room temperature and slowly add 14.4g of sodium chloride into the reaction flask, and react with the solution in the reaction flask. After the addition is complete, keep the temperature at 50°C and keep it warm for 1 hour. Dilute to 1000ml of water, and use mineral acid, such as hydrochloric acid (HCl) or concentrated sulfuric acid (H 2 SO 4 ), the acid-base value (p H value) of this reaction solution is adjusted to 3. Next, use 1000ml of ethyl acetate to extract the reaction intermediate after pH adjustment three times, combine the extracts obtained after three extractions with ethyl acetate solution, wash with saturated brine, and wash with anhydrous Sodium sulfate is dried, and the ethyl acetate in the extract is removed b...
Embodiment 3
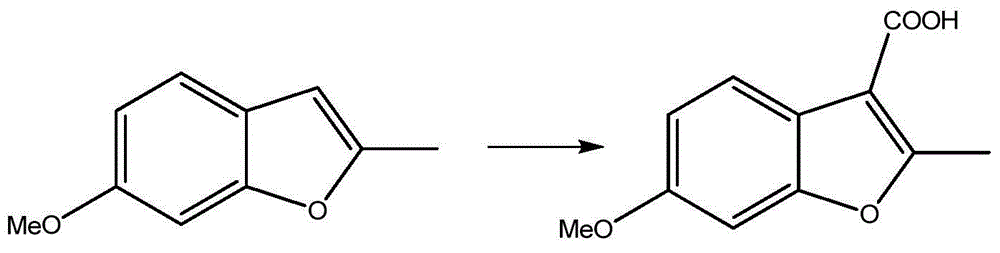
[0020] Embodiment 3: prepare 2-methyl-3-carboxy-6-methoxybenzofuran
[0021]
[0022]Add 1250ml of dichloromethane and 80.9g of aluminum chloride into a 2L reaction flask, stir in an ice bath, and cool the temperature of the reaction solution to a temperature ranging from 0°C to minus 15°C. Next, 77 g of oxalyl chloride was added to the reaction solution in the reaction flask, and after stirring for 30 minutes, the second product obtained in the aforementioned Example 2, 30 g of 2-methyl-6-methoxybenzofuran, was started to be added dropwise to the reaction flask. In the liquid, after the dropwise addition is completed, a reaction intermediate is formed, remove the ice bath, and increase the reaction temperature to 25°C-30°C, the optimal reaction temperature is 30°C, and keep the reaction intermediate at this temperature 2 hours. After the reaction, dichloromethane contained in the reaction intermediate was removed by distillation. Next, add 200 ml of water to the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com