Synthesis method of (3-cyclopropylpyridin-2-yl) methylamine hydrochloride
A technology of methylamine hydrochloride and cyclopropylpyridine, which is applied in the field of organic chemical intermediate synthesis, can solve problems such as no compound synthesis method reported in the literature, and achieve the effects of easy control, simple experimental operation and reasonable design.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
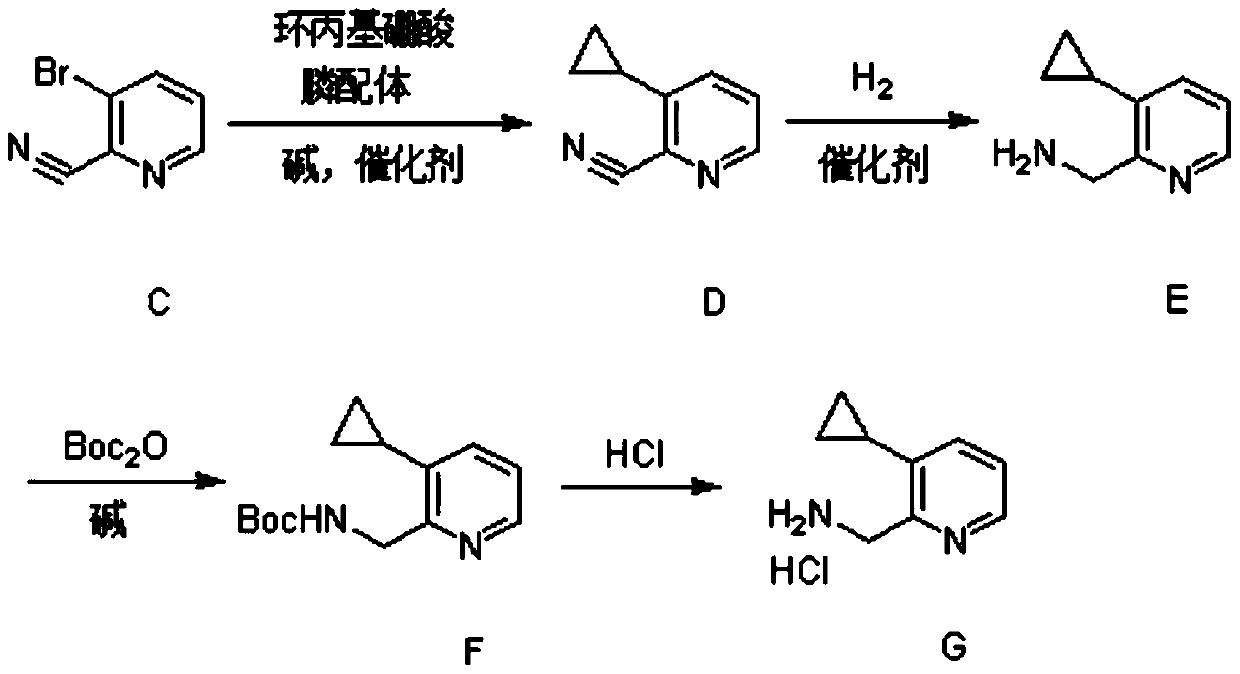
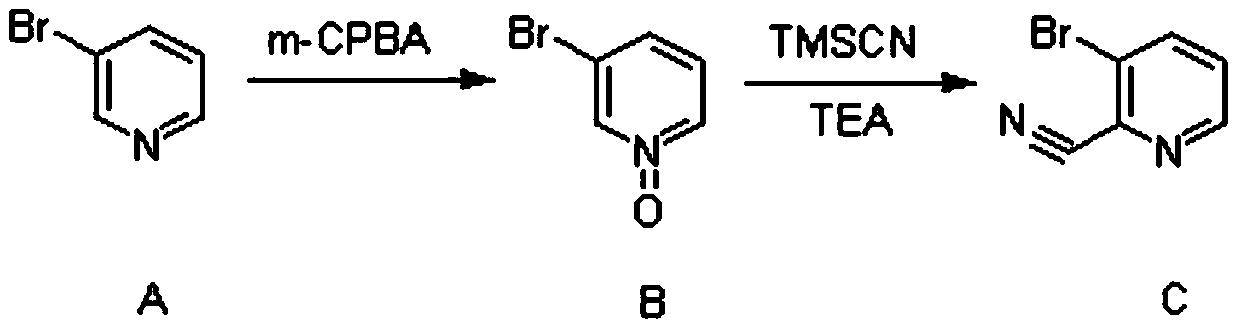
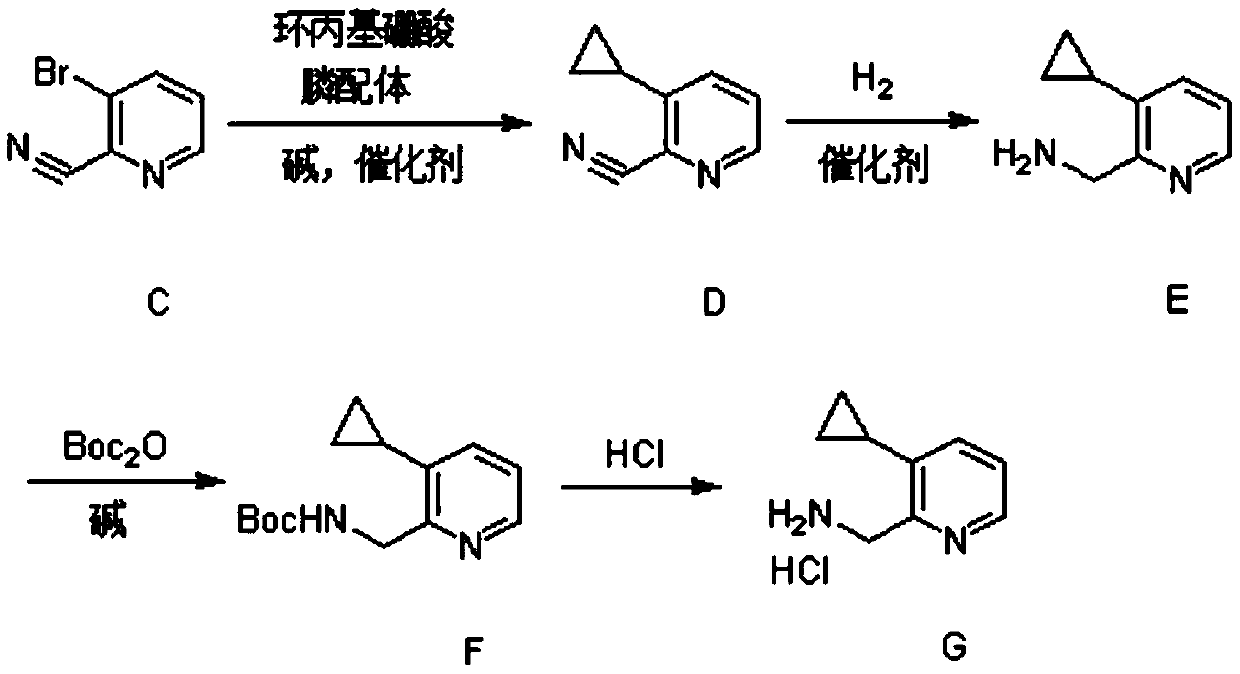
[0029] (1) Synthesis of Compound C
[0030] 3-Bromopyridine (23.7g, 150mmol, 1eq.) was dissolved in 350ml of dichloromethane, under an ice-salt bath, m-chloroperbenzoic acid (58g, 225mmol, 1.5eq.) with a mass fraction of 67% was added in batches . After the addition was complete, the reaction was carried out at 20° C. for 3 hours.
[0031] After the reaction was completed, 140 ml of saturated aqueous sodium bicarbonate solution was added under ice-cooling, and stirred for half an hour. Extracted with a mixture of dichloromethane and methanol (volume ratio 8:1), the obtained organic phase was dried, concentrated, and then purified by column chromatography to obtain 24.3g of light red oil 3-bromopyridine-N-oxide, Yield 93%.
[0032] 3-Bromopyridine-N-oxide (24.3g, 139.5mmol, 1eq.) was dissolved in 360ml of acetonitrile, under the protection of nitrogen, trimethylsilyl cyanide (41.5g, 418.5mmol, 3eq.), three Ethylamine (28.2g, 279mmol, 2eq.) was reacted at 75°C for 15 hours. ...
Embodiment 2
[0048] (1) Synthesis of Compound C
[0049]3-Bromopyridine (28.4g, 180mmol, 1eq.) was dissolved in 420ml of dichloromethane, under ice-salt bath, m-chloroperoxybenzoic acid (69.5g, 270mmol, 1.5eq. ). After the addition was complete, the reaction was carried out at 20° C. for 3 hours.
[0050] After the reaction was completed, 170 ml of saturated aqueous sodium bicarbonate solution was added under ice-cooling, and stirred for half an hour. Extracted with a mixture of dichloromethane and methanol (volume ratio 8:1), the obtained organic phase was dried, concentrated, and then purified by column chromatography to obtain 29.1g of light red oil 3-bromopyridine-N-oxide, Yield 93%.
[0051] 3-Bromopyridine-N-oxide (29.1g, 167.4mmol, 1eq.) was dissolved in 430ml of acetonitrile, under the protection of nitrogen, trimethylsilyl cyanide (49.8g, 502.2mmol, 3eq.), three Ethylamine (33.9g, 334.8mmol, 2eq.) was reacted at 75°C for 15 hours.
[0052] After the reaction was finished, aft...
Embodiment 3
[0067] (1) Synthesis of Compound C
[0068] 3-Bromopyridine (19g, 120mmol, 1eq.) was dissolved in 280ml of dichloromethane, under ice-salt bath, m-chloroperoxybenzoic acid (46.4g, 180mmol, 1.5eq.) was added in batches with a mass fraction of 67% . After the addition was complete, the reaction was carried out at 20° C. for 3 hours.
[0069] After the reaction was completed, 110 ml of saturated aqueous sodium bicarbonate solution was added under ice-cooling, and stirred for half an hour. Extracted with a mixture of dichloromethane and methanol (volume ratio 8:1), the obtained organic phase was dried, concentrated, and then purified by column chromatography to obtain 19.2g of light red oily 3-bromopyridine-N-oxide, Yield 92%.
[0070] 3-Bromopyridine-N-oxide (19.2g, 110.4mmol, 1eq.) was dissolved in 280ml of acetonitrile, under the protection of nitrogen, trimethylsilyl cyanide (32.9g, 331.2mmol, 3eq.), three Ethylamine (22.3g, 220.8mmol, 2eq.) was reacted at 75°C for 15 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com