Temozolomide intermediate compound
A technology for temozolomide and a compound, which is applied in the field of drug synthesis, can solve the problems of low yield and long route, and achieves the effects of high selectivity, easy operation, and avoiding silica gel column chromatography operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
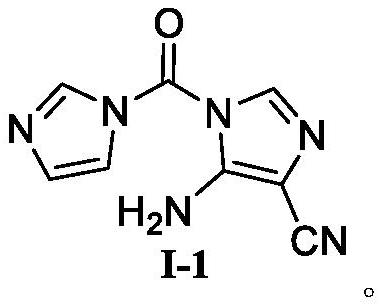
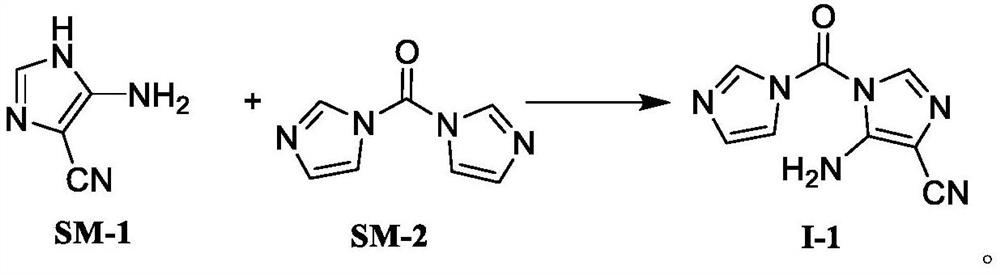
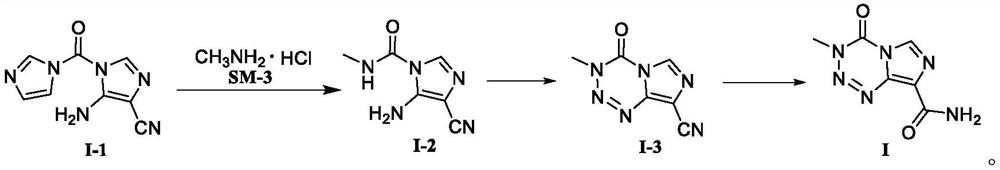
[0077] At room temperature, 5-amino-4-imidazolium cyanide (SM-1, 18.10g, 0.10mol) and carbonyldiimidazole (SM-2, 17.84g, 0.11mol) were added to 80ml of acetonitrile, and the temperature was controlled to reflux for reaction. After completion, the reaction solution was cooled to -10°C and stirred to precipitate a solid, filtered and dried to obtain compound I-1 with a yield of 85.5% and a purity of 99.86%.
Embodiment 2
[0079] At room temperature, 5-amino-4-imidazolium cyanide (SM-1, 18.10 g, 0.10 mol) and carbonyldiimidazole (SM-2, 16.22 g, 0.10 mol) were added to 80 ml of tetrahydrofuran, and the temperature was controlled to reflux for reaction. After completion, the reaction solution was cooled to -10°C and stirred to precipitate a solid, filtered and dried to obtain compound I-1 with a yield of 83.6% and a purity of 99.68%.
Embodiment 3
[0081] At room temperature, add 5-amino-4-imidazolium cyanide (SM-1, 18.10 g, 0.10 mol) and carbonyldiimidazole (SM-2, 29.19 g, 0.18 mol) into 80 ml of 1,2-dichloroethane, and control the temperature. The reaction was refluxed. After the reaction was detected, the reaction solution was cooled to -10°C and stirred to precipitate a solid, filtered and dried to obtain compound I-1 with a yield of 81.3% and a purity of 99.22%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com