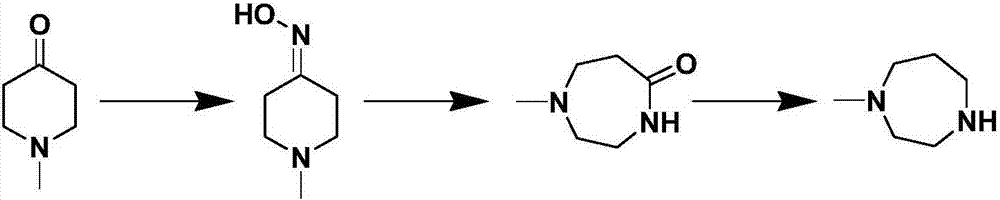
Process for preparing N-methyl homopiperazine from 2-haloethylamine compound
A technology of methylhomopiperazine and haloethylamine, which is applied in the field of preparing N-methylhomopiperazine, can solve the problems of unstable yield, violent heat release, and many wastes, and achieve high production operation safety, The effect of simple synthesis process and convenient reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0077] The method for preparing homopiperazine described in this example may further comprise the steps:
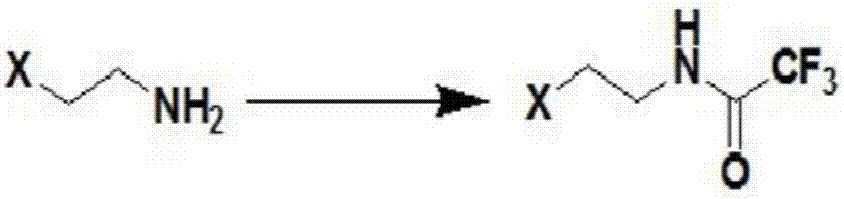
[0078] Step 1: Preparation of N-(2-chloroethyl)trifluoroacetamide
[0079]
[0080] Under stirring at room temperature, add 30L of ethanol, 3.48kg of 2-chloroethylamine hydrochloride and 4.27kg of ethyl trifluoroacetate to the 50L reaction kettle in sequence, stir well, cool to 0°C, and dropwise add 3.04kg of triethylamine , 2 hours to drop. After dripping, react at room temperature for 4 hours, and the reaction ends.
[0081] Ethanol was recovered by distillation under reduced pressure, and the residue was beaten and washed with 10 L of tap water. Filtrate, drain the filter cake, and dry in an oven at 50°C for 4 hours to obtain 4.92Kg of a white low-melting solid product with a yield of 93.4% and a melting point of 59-60°C.
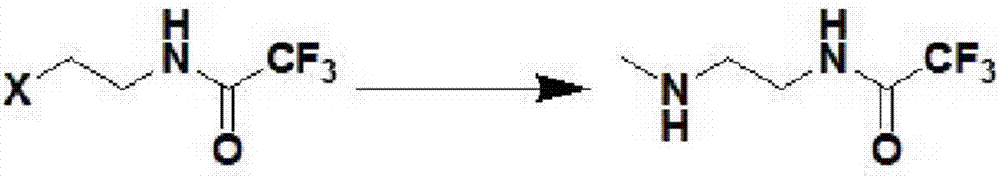
[0082] Step 2: Preparation of N-methyl-N'-trifluoroacetylethylenediamine
[0083]
[0084] Under stirring at room temperature, 25L of ace...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com