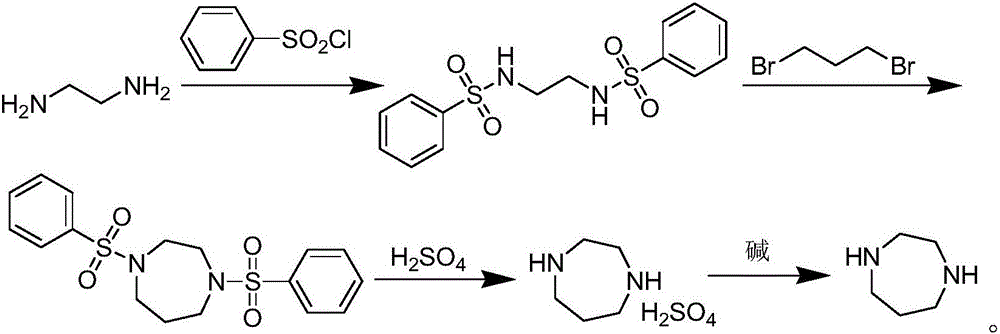
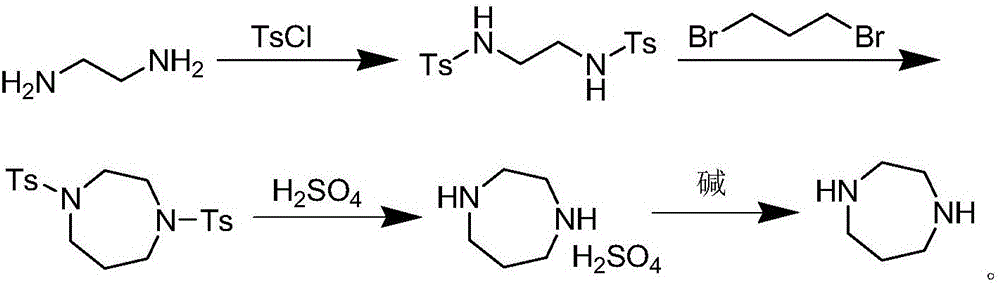
Method for preparing homopiperazine by utilizing ethyl trifluoroacetate
A technology for ethyl trifluoroacetate and ditrifluoroacetyl homopiperazine, which is applied in the field of preparing homopiperazine, can solve the problems of health hazards of operators, high reaction temperature, and many three wastes, etc., and achieves high production and operation safety. , The effect of simple reaction operation and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
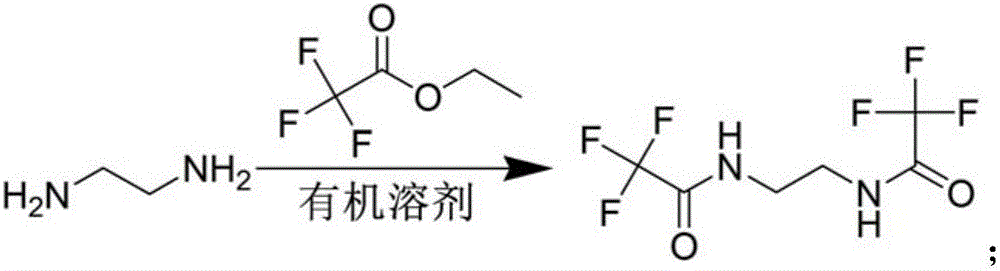
[0068] Step 1, the preparation of ditrifluoroacetylethylenediamine
[0069] Under stirring at room temperature, 20 L of ethanol and 10 kg of ethyl trifluoroacetate were successively added into the 50 L reaction kettle. Then it was cooled to 0° C., and 2.1 kg of ethylenediamine was added dropwise thereto, and the dropwise was completed in 4 hours. Due to the intense heat release during the dropwise addition, white solids were continuously generated. After dripping, stirring was continued for 1 hour at room temperature, and the reaction was completed.
[0070] After the reaction, filter, drain the filter cake, and dry in an oven at 60° C. for 4 hours to obtain 8.5 Kg of a white solid product, bistrifluoroacetylethylenediamine, with a yield of 96.5%.
[0071] Wherein, the chemical reaction formula of above-mentioned preparation process is:
[0072]
[0073] Step 2, the preparation of ditrifluoroacetyl homopiperazine
[0074] Under stirring at room temperature, 40 L of acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com