Synthetic process for Vonoprazan fumarate
A technology of fumaric acid and fumarate, which is applied in the field of medicine, can solve the problems of cumbersome refining steps, increased production costs, and low product purity, and achieve high product purity, cost saving, and high removal efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
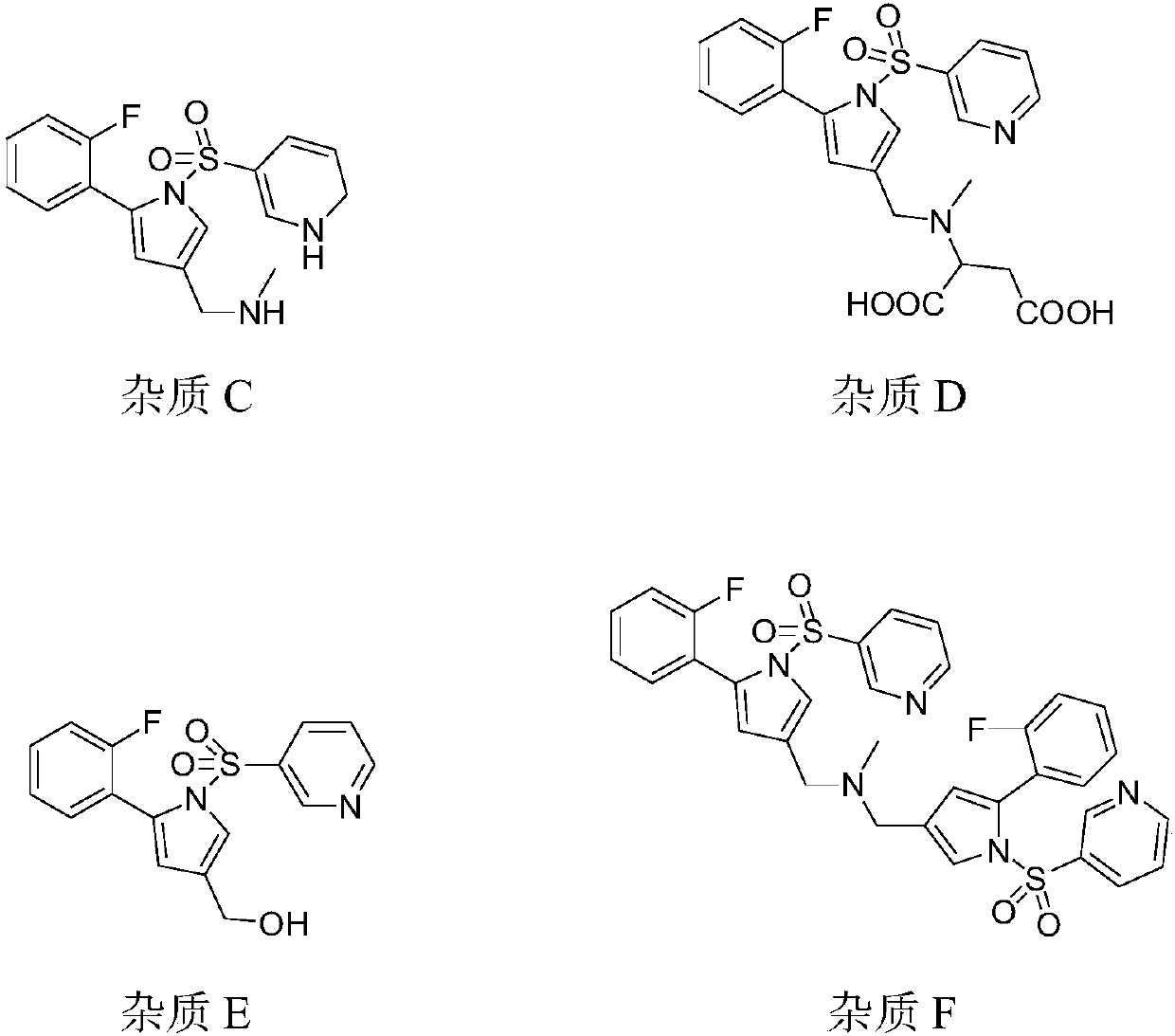
[0058]Put 10.00g of 5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde, 1.29g of 4-dimethylaminopyridine (DMAP), 7.49g of triethylamine and 1L of dichloromethane into the reactor, stir and cool down, Add 11.26g of pyridine-3-sulfonyl chloride dropwise, stir and react for 2 hours after dropping, add 20ml of water to quench the reaction, wash in turn with 20ml of water, 20ml of 20% sodium chloride solution by mass percentage, distill, add 70ml of 75% ethanol solution Heat to dissolve, cool down to crystallize, filter, wash, and dry to obtain 15.12 g of 1-(3-pyridinesulfonyl)-5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde. Yield 86.60%.
[0059] Put 10.00g 1-(3-pyridinesulfonyl)-5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde (0.0303mol) and 50ml methanol into the reactor, stir, add 1.63g methylamine (0.05258 mol), stirred and reacted at 5°C for 1 hour, cooled to 0°C, added 0.77g sodium borohydride (0.02026mol), and continued to keep warm for 60 minutes after adding, quenched the reaction ...
Embodiment 2
[0063] Put 10.00g 1-(3-pyridinesulfonyl)-5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde (0.0303mol) and 50ml methanol into the reactor, stir, add 1.80g methylamine (0.05806 mol), stirred and reacted at 10°C for 2 hours, cooled to -5°C, slowly and uniformly added 0.64g sodium borohydride (0.01684mol), continued to keep warm for 60 minutes after the addition was complete, added dropwise 20ml of water to quench the reaction, and stirred for 35 minutes , methanol was distilled off under reduced pressure, 120ml of ethyl acetate and 20ml of water were added to the residue, the liquid was separated, the organic layer was taken for washing, and dilute hydrochloric acid was added dropwise under cooling to pH 4, stirred at room temperature for 30 minutes, and the mass percentage concentration was 10% chloride Sodium solution, stirred and crystallized, filtered, and dried to obtain 10.46 g of vonoprazan hydrochloride. Yield 90.49%, HPLC purity 98.04%, impurity content: impurity A is 0.37%,...
Embodiment 3
[0067] Put 10.00g 1-(3-pyridinesulfonyl)-5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde (0.0303mol) and 100ml methanol into the reactor, stir, add 1.51g methylamine, 20 Stir the reaction at ℃ for 1 hour, lower the temperature to -10 ℃, slowly and uniformly add 0.58g sodium borohydride (0.01526mol), continue the heat preservation reaction for 90 minutes after the addition, drop water to quench the reaction, stir the reaction for 40 minutes, and remove methanol by distillation under reduced pressure , add 120ml of ethyl acetate and 20ml of water to the residue, separate the layers, take the organic layer and wash it, add dilute hydrochloric acid dropwise to pH 2 under cooling, stir at room temperature for 30 minutes, add saturated sodium chloride solution, stir and crystallize, filter, and dry. Recrystallization gave 8.69 g of Vonorazan hydrochloride. Yield 75.18%, HPLC purity 99.60%, impurity content: impurity A not detected, impurity B 0.028%, impurity C 0.061%, impurity D not d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com