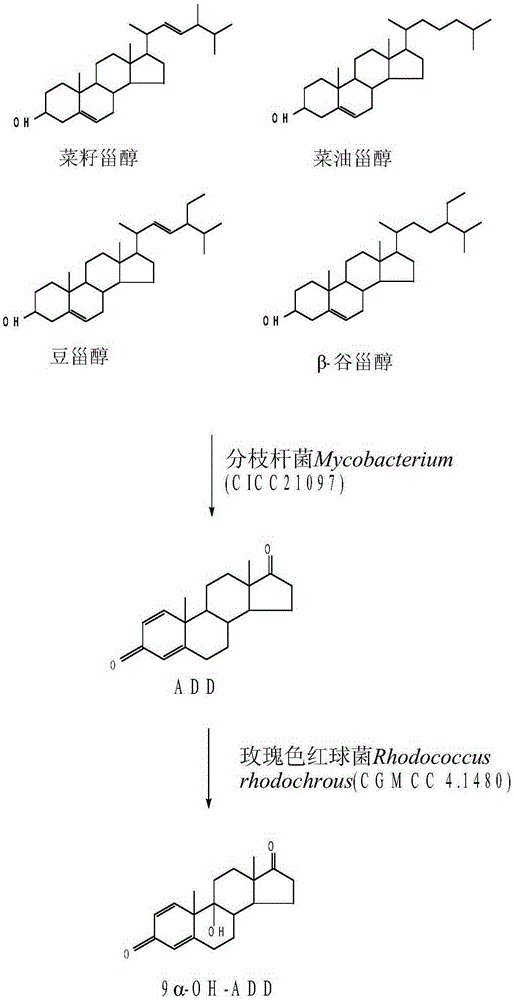
Method for preparing 9 alpha-hydroxy-androstane-1,4-diene-3,17-dione
A technology of androstane and diene, applied in the field of microbial pharmacy, can solve problems such as low conversion rate, difficult separation and purification, and achieve the effects of improving utilization efficiency, improving biological efficiency and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Mycobacterium Mycobacterium (CICC21097) and Rhodococcus rhodochrous (CGMCC4.1480) were cultured on slant and seeds to obtain the strains required for fermentation. 1. Preparation of mycobacterium Mycobacterium (CICC21097) slant strain and seed solution
[0031] (1) Incline cultivation
[0032] Slant medium: peptone 5.0g / L, beef extract 3.0g / L, sodium chloride 5.0g / L, glucose 10.0g / L, agar 20.0g / L, cultured at 29°C for 5-7 days.
[0033] (2) Seed cultivation
[0034] Seed medium: glucose 15.0g / L, peptone 5.0g / L, beef extract 3.0g / L, sodium chloride 5.0g / L, magnesium sulfate heptahydrate 2.5g / L, ammonium dihydrogen phosphate 3.5g / L, phosphoric acid Dipotassium hydrogen 1.0g / L, potassium dihydrogen phosphate 1.0g / L, pH 7.0, sterilized by moist heat at 121°C for 30min.
[0035] The bacterial strain cultivated in step (1) was inoculated under sterile conditions with an inoculation loop into a 250mL shake flask containing 50mL seed medium, and cultivated on a rotary shaker...
Embodiment 2
[0048] Mycobacterium Mycobacterium (CICC21097) and Rhodococcus rhodochrous (CGMCC4.1480) strains were prepared into seed liquid (the method is the same as in Example 1), and then fermented.
[0049] Fermentation medium: phytosterol 35.0g / L, ethanol 31.0g / L, polyethylene glycol 9.0g / L, glucose 20.0g / L, ammonium dihydrogen phosphate 3.0g / L, ferric ammonium citrate 0.05g / L, Magnesium sulfate heptahydrate 2.5g / L, dipotassium hydrogen phosphate 1.0g / L, potassium dihydrogen phosphate 1.0g / L, beet molasses 6.0g / L, pH7.0.
[0050] The fermenter containing the fermentation medium was sterilized by high-pressure steam at 121° C. for 15 minutes.
[0051] At the beginning of the culture, a compound solubilizing agent was added to the medium: ethanol 31.0 g / L, polyethylene glycol 9.0 g / L. Carry out inoculation again, the inoculum size of mycobacterium MycobacteriumCICC (21097) is 12.0%, inoculate Rhodococcus rhodochrous after mycobacterium transformation culture 24h, the inoculum size of ...
Embodiment 3
[0054] Mycobacterium Mycobacterium (CICC21097) and Rhodococcus rhodochrous (CGMCC4.1480) strains were used to prepare seed liquid (the method is the same as in Example 1), and then fermented.
[0055] Fermentation medium: phytosterol 35.0g / L, glucose 22.0g / L, ammonium dihydrogen phosphate 3.0g / L, ferric ammonium citrate 0.05g / L, magnesium sulfate heptahydrate 2.0g / L, dipotassium hydrogen phosphate 1.0g / L, potassium dihydrogen phosphate 1.0g / L, beet molasses 6.0g / L, pH7.0.
[0056] The fermenter containing the fermentation medium was sterilized by high-pressure steam at 121° C. for 15 minutes.
[0057] At the beginning of the culture, a compound solubilizing agent was added to the medium: ethanol 30.0g / L, polyethylene glycol 9.0g / L. Carry out inoculation again, the inoculum size of mycobacterium MycobacteriumCICC (21097) is 13.0%, inoculate Rhodococcus rhodochrous after mycobacterium transformation culture 48h, the inoculum size of Rhodococcus rhodochrous (CGMCC4.1480) is 17....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com