Adriamycin-loaded PEGylated peptide dendrimer targeted drug delivery system and preparation method thereof
A dendrimer, targeted drug delivery technology, used in pharmaceutical formulations, medical formulations with inactive ingredients, and medical formulations containing active ingredients, etc., to achieve high biological safety, reduce the risk of overflowing the tumor site, The effect of improving biosafety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
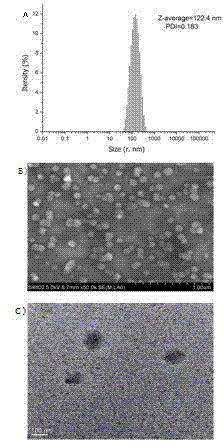
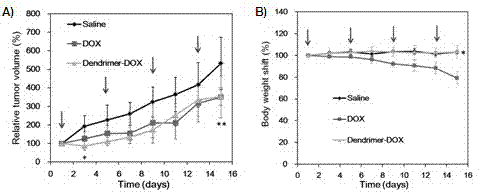
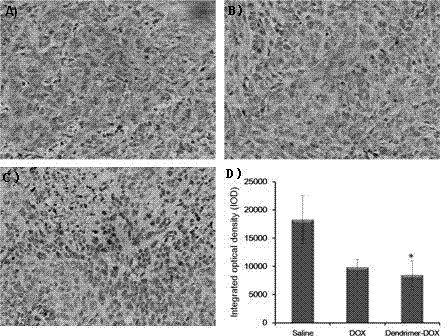
[0061] PEGylated peptide dendrimer targeted drug delivery system loaded with doxorubicin, the drug delivery system is a conjugate of anti-tumor drug doxorubicin, targeting functional factor GFLG and PEGylated polypeptide dendrimer, Its structure is as Figure 7 :
[0062] Conjugate I: n=10, m=1 PEGylated third-generation lysine dendrimer-doxorubicin conjugate
[0063] Conjugate II: PEGylated third-generation lysine dendrimer-doxorubicin conjugate with n=1, m=1
[0064] Conjugate III: PEGylated 4th generation lysine dendrimer-doxorubicin conjugate with n=1, m=1
[0065] Conjugate IV: PEGylated 5th generation lysine dendrimer-doxorubicin conjugate with n=2, m=1
[0066] Conjugate V: PEGylated third-generation lysine dendrimer-doxorubicin conjugate with n=5, m=6
[0067] Conjugate VI: a=10, b=1 PEGylated 4th generation glutamic acid dendrimer-doxorubicin conjugate
Embodiment 2
[0069] The preparation method of the GFLG-based PEGylated polypeptide dendrimer drug delivery system includes firstly introducing a methyl vinyl group into the conjugate of doxorubicin and GFLG to obtain the conjugate MA-GFLG-DOX for use; As a carrier, the peptide dendrimer is alkynylated, and a tritylthio group is introduced on the dendrimer to obtain an alkynyltritylthio dendrimer. After removing the trityl group, it is coupled with MA-GFLG-DOX undergoes a coupling reaction to obtain a conjugate of alkynylated dendrimer and doxorubicin, which is finally PEGylated to obtain the finished product.
Embodiment 3
[0071] The specific operation steps of the preparation method of the present invention are as follows:
[0072] A preparation of MA-GFLG-DOX conjugates;
[0073] Using Boc-GFLG-OMe as raw material, react with methyl vinyl chloride to prepare MA-GFLG-OH, then react MA-GFLG-OH with N,N-diisopropylethylamine and tetrahydrothiazole-2-thione , after the reaction, the reaction product, doxorubicin hydrochloride, and pyridine were dissolved in anhydrous dimethyl sulfoxide, and reacted in the dark, and the reaction product was MA-GFLG-DOX conjugate;
[0074] Synthesis of B Alkynylated Dendrimer
[0075] The dendrimers protected by Boc and Cbz groups are used as raw materials to react with Pb / C in a hydrogen atmosphere. After the reaction, the reaction product is dissolved in DMF, and N,N-diisopropylethylamine is added to the system , hexynoic acid, benzotriazole-N, N, N', N'-tetramethyluronium hexafluorophosphate, 1-hydroxybenzotriazole, react under the protection of nitrogen, and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com