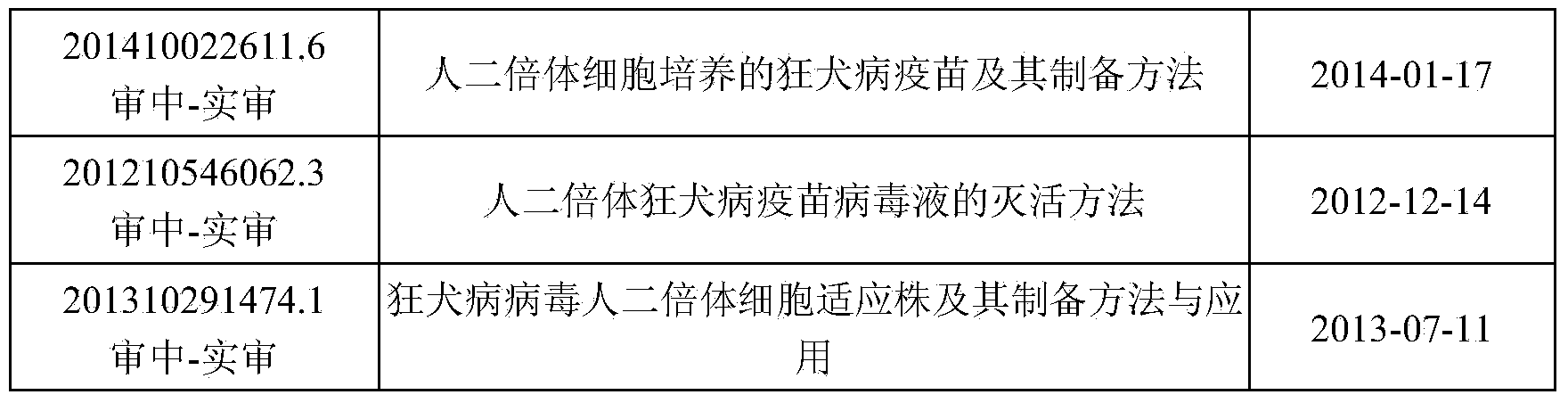
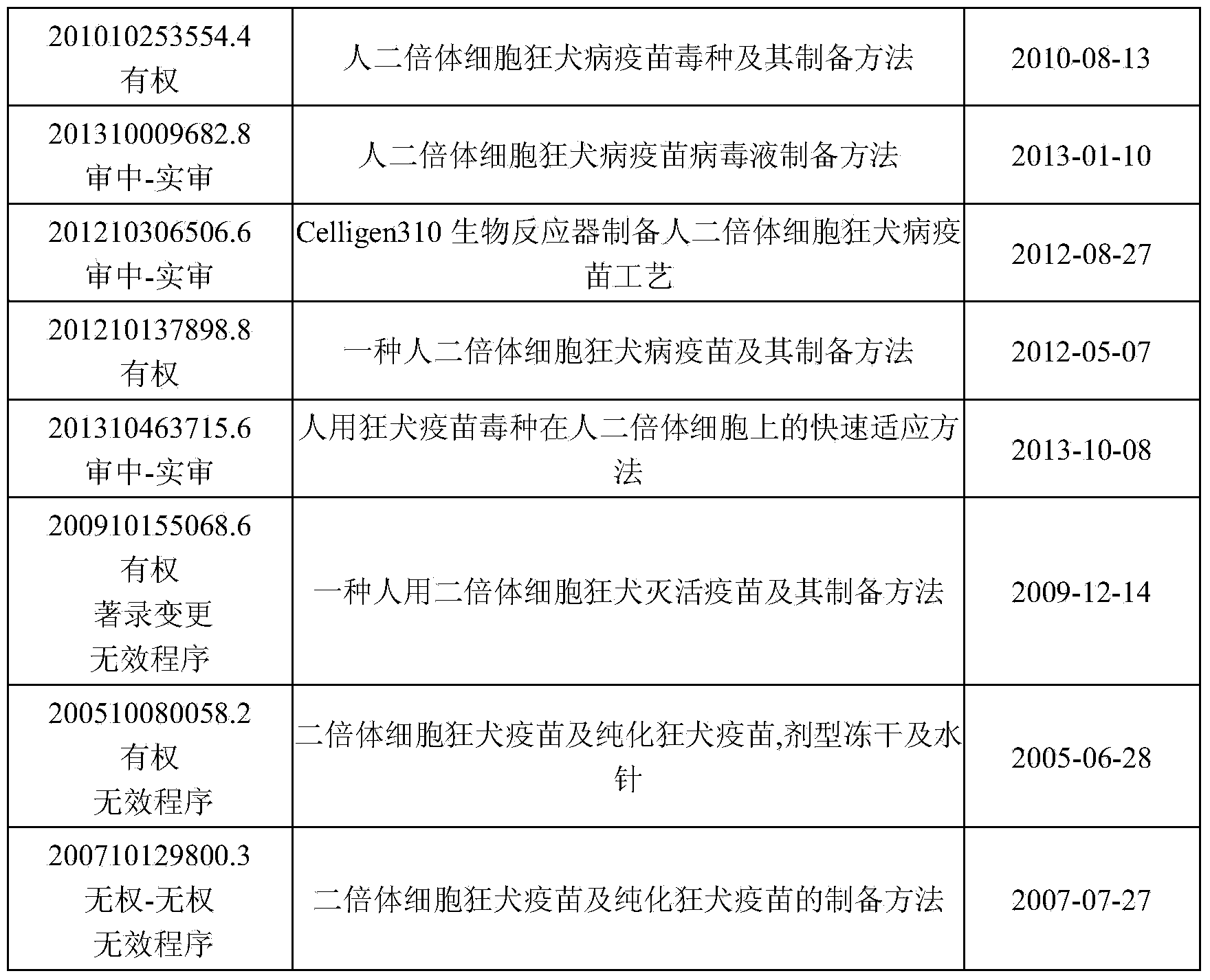
Rabies virus SNK-CTN strain and application thereof
A technology of rabies virus and virus strains, which is applied in antiviral agents, viruses/bacteriophages, medical preparations containing active ingredients, etc., can solve the problems of difficult vaccine extraction, unstable production, and many impurities, and achieve good protection effect. Reduce the steps of extraction and purification, the effect of simple vaccine extraction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1 Adaptation of rabies virus CTN strain on MRC-5 cells
[0052] Take the cryopreservation tube containing MRC-5 cells, put it into a 40°C water bath to melt quickly, then add it to the MEM cell culture medium pre-warmed to 37°C, culture it at 37°C, and grow into a single layer after 3-4 days After digestion with 0.25 trypsin, inoculate CTN-1V5 strain suspension at 0.01-1.0 MOI dose, culture at 37°C for 2-3 days, replace with maintenance solution containing 2-4% bovine serum, and cultivate at 33-35°C for 3-5 days The virus liquid was harvested, frozen at -70°C, and prepared into a virus suspension.
[0053] According to the above method, the harvested virus suspension was continuously uploaded to MRC-5 cells for 30 generations, and finally a rabies virus SNK-CTN strain with good adaptability to MRC-5 cells was obtained.
[0054] The rabies virus SNK-CTN strain that the present invention obtains carries out following observation and experiment:
[0055] (1) S...
Embodiment 2
[0057] Example 2 Application of SNK-CTN strain to prepare diploid cell rabies vaccine
[0058] Take human embryonic lung diploid cells (MRC-5) cultured in monolayer cell flasks, digest the cells with 0.25% trypsin, amplify the cells to the cell factory or bioreactor, add MEM cell culture medium, and the cells The medium was inoculated with SNK-CTN rabies virus at 0.05-0.IMOI, cultured at 37°C for 3 days, discarded the culture medium and replaced with maintenance medium. Place the culture at 35°C for 3 to 15 days, and harvest the virus liquid for 2 to 3 times according to the pathological changes of the cells in the cell factory; harvest the virus multiple times according to the indicators monitored by the reactor and the pathological changes of the cells as the virus harvest liquid.
[0059] The harvest liquid was inactivated by adding 1:4000 β-propiolactone under sterile conditions for 24 hours. After passing the inactivation test, the inactivated rabies virus liquid is conc...
example 3
[0062] Example 3 SNK-CTN strain prepares the quality inspection of diploid cell rabies vaccine
[0063] 1) Determination of virus titer
[0064] 10-fold serial dilution of the sample, intracerebral challenge of mice, each 0.03ml per dilution intracerebral inoculation of at least 6 mice weighing 11-13g, observed for 14 days, the virus titer should not be less than 7.0lg LD 50 / ml.
[0065] 2) Determination of antigen content
[0066] The purified anti-rabies virus polyclonal antibody was used to coat the enzyme-linked reaction plate and labeled horseradish peroxidase (HRP), and the double-antibody sandwich principle was used to quantitatively detect the antigen content in the rabies vaccine by using a two-step reaction mode. , should not be less than 0.4IU / dose.
[0067] Antigen content of human rabies vaccine and results of virus titer of corresponding virus harvest fluid
[0068] Test items
SNK-CTN Harvest Solution 1
SNK-CTN Harvest Solution 2
SNK-CT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com