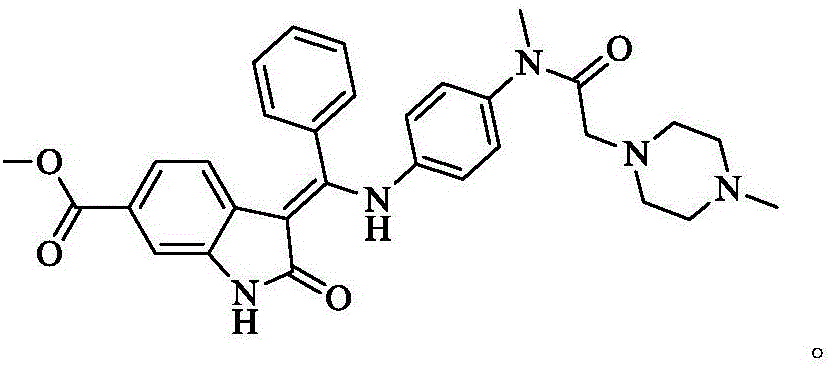
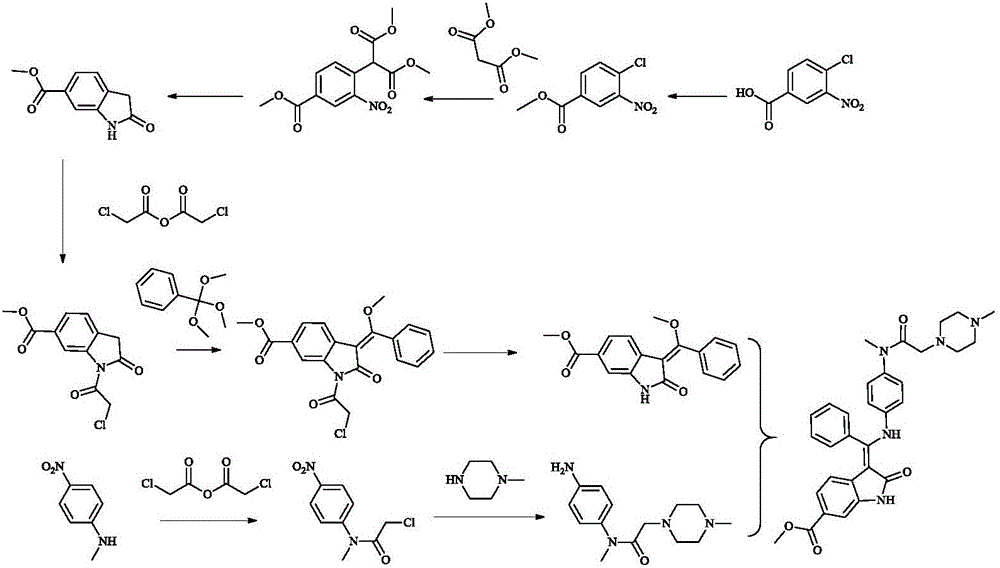
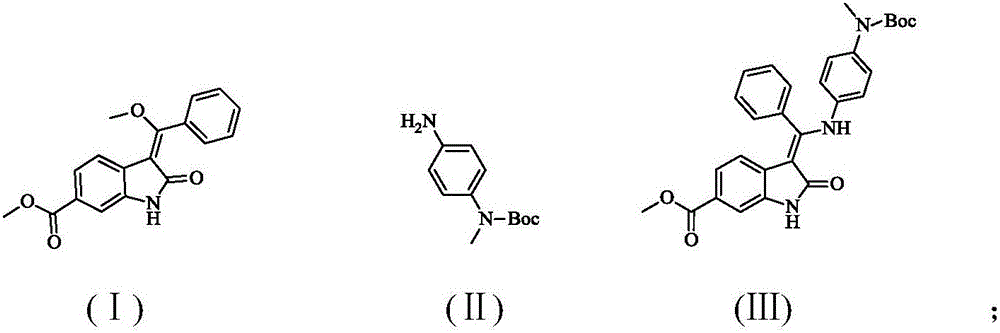
A synthetic method of Nintedanib and an intermediate of Nintedanib
A synthesis method and technology of nintedanib, applied in the field of medicine and chemical industry, can solve the problems of low reaction efficiency of chloroacetic anhydride, unstable route repeatability, unfavorable scale-up production, etc., to reduce potential safety hazards, rational route design, and save capital. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1: the preparation of compound 4-chloro-3-nitrobenzoic acid methyl ester
[0065] After suspending 200g (1mol) of 4-chloro-3-nitrobenzoic acid in methanol, add 59g (490mmol) of thionyl chloride within 15min, heat and reflux for reaction, react at 70°C for 3 hours and then cool to 5°C, the product After centrifugal separation and drying at 45° C., 189.2 g of a solid, namely the target compound, was obtained with a yield of 88.5%.
Embodiment 2
[0066] Embodiment 2: the preparation of compound 2-oxindole-6-methyl carboxylate
[0067] Add 128.7 g (974 mmol) dimethyl malonate to a hot melt (75° C.) of 107.3 g (974 mmol) sodium tert-amylate in 350 mL N-methyl-2-pyrrolidone; add at 75° C. 100g (463mmol) methyl 4-chloro-3-nitrobenzoate in 250mL of N-methyl-2-pyrrolidone. Stir at about 75°C for 1.5 hours, and cool to 20°C, acidify the mixture to pH=1 with 1000mL dilute hydrochloric acid, filter to obtain 137.6g of solid, dissolve the solid in 880mL of acetic acid, add 10% palladium carbon catalyst at 45°C Catalytic hydrogenation under low temperature; after the hydrogenation stopped, the reactant was heated to 115°C for 2 hours, the catalyst was filtered off, and 1800mL of water was added at about 50°C, the reaction product was cooled to 5°C, centrifuged, and Dry at 50°C to obtain 69.6 g of solid, namely the target compound, with a yield of 82.6%.
Embodiment 3
[0068] Embodiment 3: Preparation of compound 1-chloroacetyl-2-oxoindoline-6-formic acid methyl ester
[0069] At room temperature, 19.1g (100mmol) of 2-oxindole-6-methyl carboxylate was suspended in 60ml of toluene, and then 25.6g (150mmol) of chloroacetic anhydride was added, and the mixture was heated under reflux at 120°C for 3 hours, then cooled to 80°C, add 30ml of methylcyclohexane within 30min, and slowly lower to room temperature; separate the mother liquor, wash the solid with frozen methanol, and dry to obtain 25.36g of white crystals, namely the target compound, with a yield of 95%.
[0070] 1 H-NMR (400MHz, DMSO-d 6 )δ: 8.66(m, 1H); 7.86(d, J=8.0Hz, 1H); 7.52(d, J=8.0Hz, 1H); 4.99(s, 2H); 4.27(s, 3H); s, 2H). MS:m / z 268(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com