Univalent and bivalent gene engineered subunit vaccine for hand-foot-and-mouth disease and preparation method thereof
A subunit vaccine, hand, foot and mouth disease technology, applied in the field of biopharmaceuticals, can solve the problems of undeveloped vaccines with commercial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
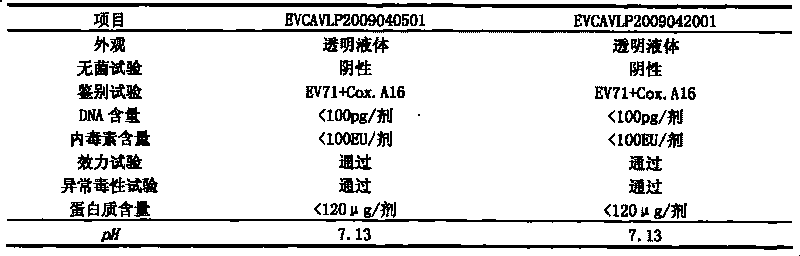
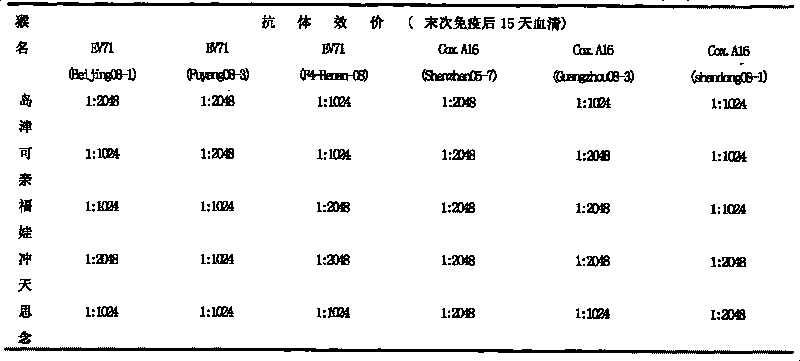
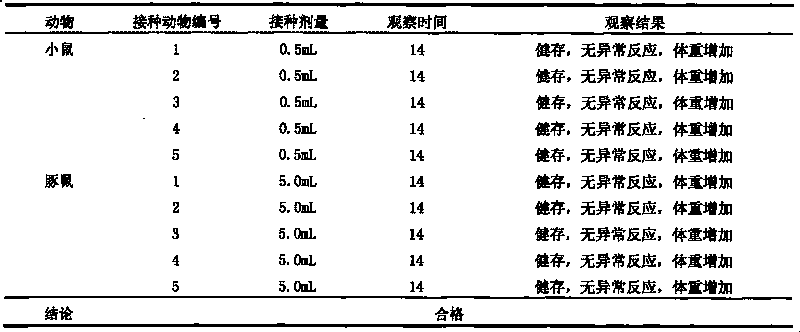
[0044] Embodiment 1: Preparation of hand, foot and mouth disease subunit vaccine of the present invention
[0045] 1. Preparation of recombinant baculovirus Bac-EV71-P1-3CD and Bac-Cox.A16-P1-3CD
[0046] Cells: African green monkey kidney cell line (Vero cell line) and insect cell line Sf-9 were used.
[0047] Viruses: H13-Henan-08 was used for strain EV71, and S10-Shandong-08 was used for strain Cox.A16.
[0048] Cell culture: resuscitate the cells, resuscitate the cells in one ampoule into one cell culture flask, after 4-6 days of culture, spread out 4 cell culture flasks according to the split ratio of 1:4.
[0049] Virus inoculation: after cell culture for 3 to 5 days, discard the cell culture supernatant, and inoculate with HFMD virus. The virus inoculation amount is 0.1 MOI, the culture temperature is 37° C., the culture time is 30-40 hours, and the virus maintenance solution is DMEM medium containing 2% fetal bovine serum.
[0050] Harvest virus: When the cells have...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com