CA-193 virus strain and application thereof to preparation of inactivated vaccine
A Coxsackie virus and virus strain technology, applied in the direction of viruses, antiviral agents, virus antigen components, etc., can solve the problems of slow replication, increased difficulty in the prevention and control of severe HFMD, and insufficient research on CA16 to achieve immunogenicity. Good sex and immune protection, good immune protection effect, perfect preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1CA-193
[0024] Isolation and identification of embodiment 1CA-193 virus strain
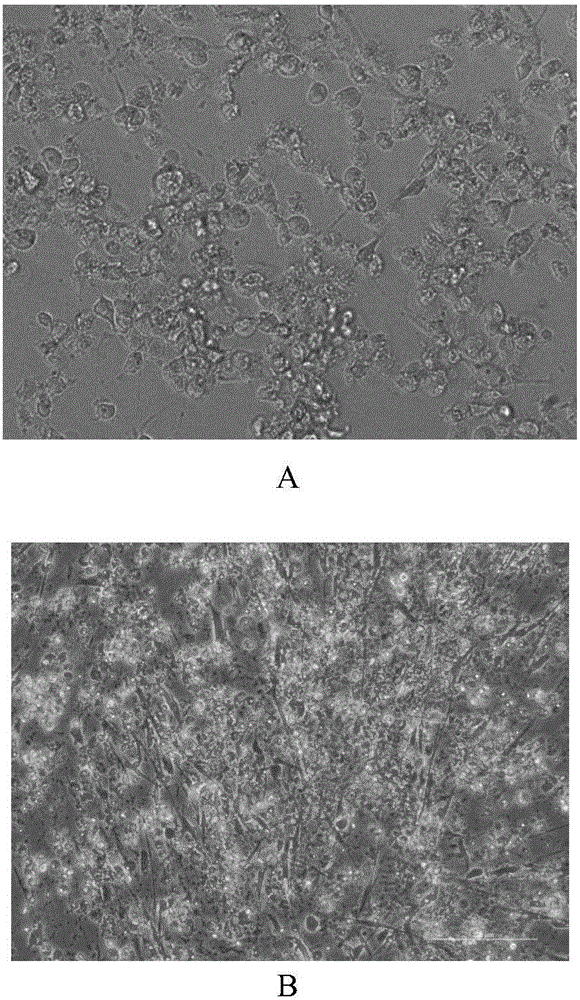
[0025] In 2010, throat swabs and herpes fluid samples were obtained from a 12-month-old male patient with hand, foot and mouth disease in the Sixth People's Hospital of Hangzhou. The samples were filtered through a 0.22 μm filter membrane, and the filtrate was inoculated into a single layer of green monkey kidney Passage cells (Vero, purchased from the American Type Culture Collection (ATCC CCL-81)) 25cm 2 In the square bottle, collect the cell suspension after more than 80% of the Vero cells have lesions, freeze and thaw three times, centrifuge at 1000rpm for 10 minutes, take the supernatant as the virus liquid, and take 1ml of the virus liquid to inoculate the Vero cells (25cm 2 square bottle), and after repeated passage three times, the CA-193 virus strain was obtained by the plaque method, and was determined to be the original strain of the first generation. figure 1 Middle A is the situation of Vero...
Embodiment 2
[0052] The preparation of embodiment 2 vaccine
[0053] (1) Recovery and culture of diploid cells
[0054] The cryopreserved human embryonic lung diploid fibroblast MRC-5 (purchased from the American Type Culture Collection (ATCCCCL-171)) was thawed in a 37°C water bath, and inoculated into MEM medium containing 20% fetal bovine serum ( Invitrogen), transfer to cell culture incubator, 37 ℃, 5% CO 2 After culturing until the cells cover the surface of the culture flask, digest with 0.25% trypsin for 3-5 minutes, gently pipette the cells to a single-cell suspension, subculture at a ratio of 1:2, pass to the sixth generation, and obtain the sixth generation Generation of MRC-5 cells.
[0055] (2) Virus cultivation and amplification
[0056] Preparation of CA-193 virus seed: wait for step (1) the sixth generation of MRC-5 cells to cover 75cm 2 Culture flask, discard the culture solution, inoculate the CA-193 virus strain in Example 1 to MRC-5 cells, the inoculum amount is MO...
Embodiment 3
[0062] The mensuration of embodiment 3 vaccine potency
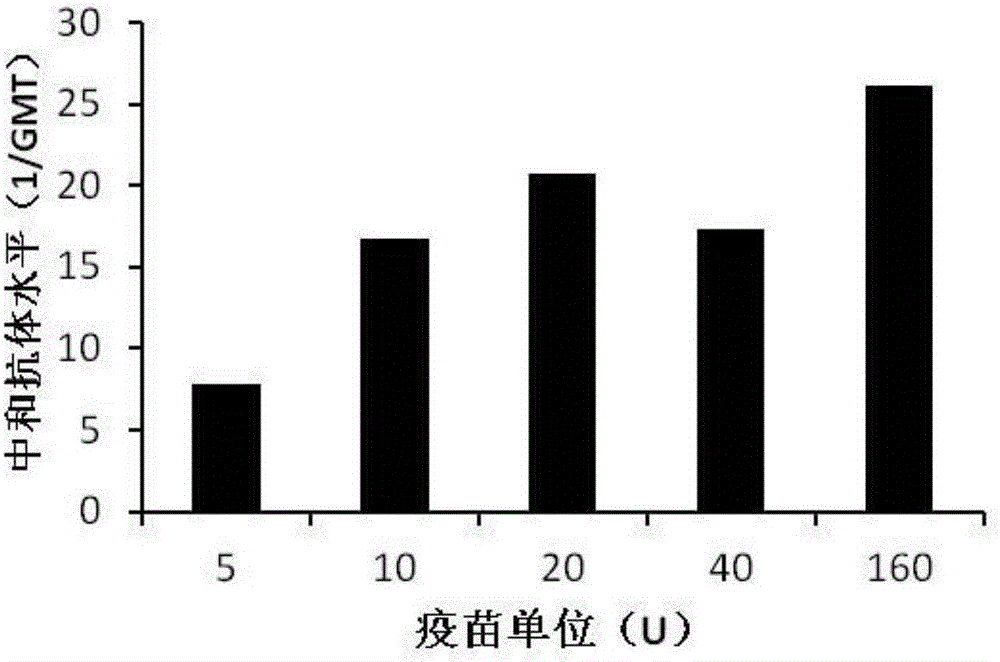
[0063] The titer of the pure vaccine prepared in Example 2 was determined by conventional ELISA method. 100 μl of vaccine diluted with PBS was added dropwise to a 96-well plate, and coated overnight at 4°C. The specific anti-CA16 polyclonal antibody was diluted with PBS at a concentration of 1:2000-1:10000 and added to a 96-well plate, incubated at 37°C for 60 minutes, and then incubated with HRP-linked secondary antibody (1:5000-1:10000, diluted in PBS) for 1 hour , Detect the absorbance at a wavelength of 450nm, if the absorbance is greater than 0.1, it is considered positive, and the vaccine titer with the maximum positive dilution is set as 400U.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com