Coxsackie virus A6 type and A10 type combined detection kit and application of same
A coxsackie virus and joint detection technology, applied in the field of biomedicine, can solve the problems of inability to distinguish enteroviruses, achieve the effect of saving specimens and improving detection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1. Coxsackievirus A6 and A10 type combined detection kit detects Coxsackievirus type A6 and A10 in the standard product
[0028] 11. Prepare nucleic acid fluorescent PCR detection mixture:
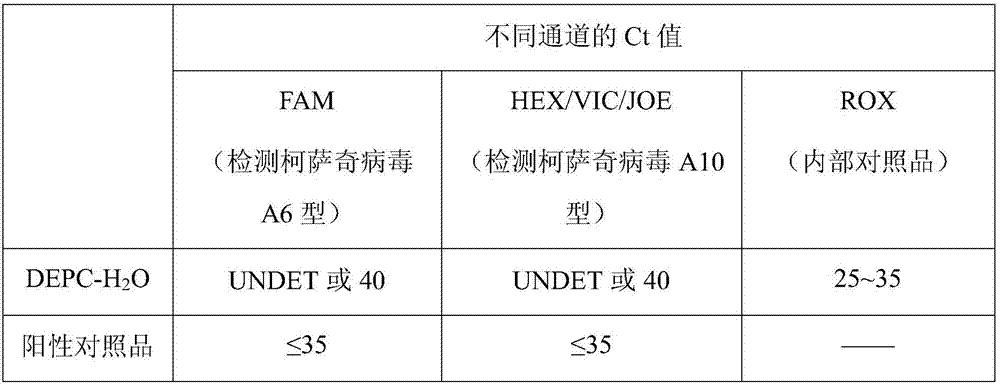
[0029]Take Coxsackie virus A6 type upstream primer 0.5 μl / test, downstream primer 0.5 μl / test, probe 0.1 μl / test; Coxsackie virus A10 type upstream primer 0.5 μl / test; downstream primer 0.5 μl / test; probe 0.1μl / test; internal control probe 0.1μl / test; real-time quantitative PCR (Realtime-PCR, RT-PCR) MIX 12.5μl / test; process water 3.2μl / test; And A10 nucleic acid fluorescent PCR detection mixture.
[0030] Wherein, the nucleotide sequence of the Coxsackievirus A6 upstream primer is shown in SEQ ID NO.1, specifically: 5'-TGTCGGTGAATAGRTTCTA-3'; the nucleotide sequence of the Coxsackievirus A6 downstream primer As shown in SEQ ID NO.2, specifically: 5'-CTGTGTCATTTAGYACRTCA-3'; the structure of the Coxsackievirus A6 probe is 5'-the first fluorescent reporter group-the first b...
Embodiment 2
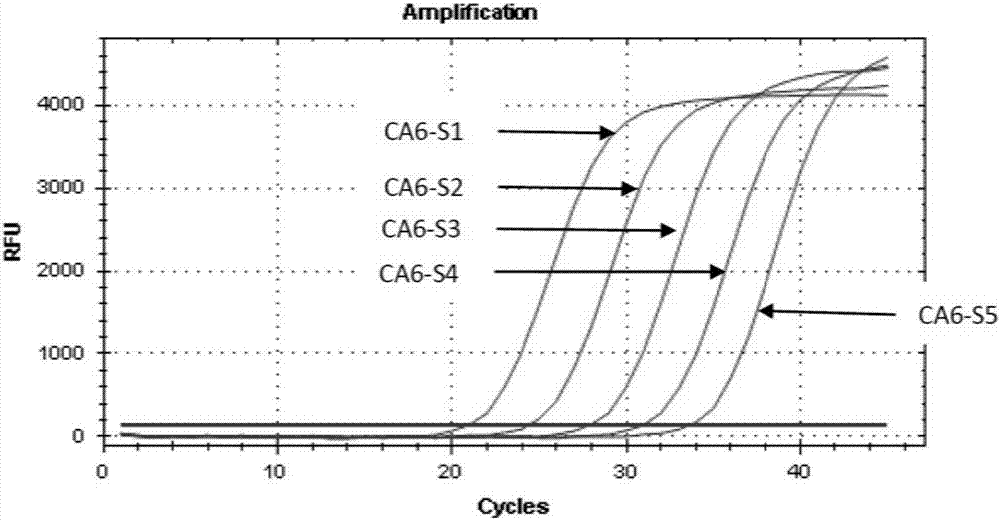
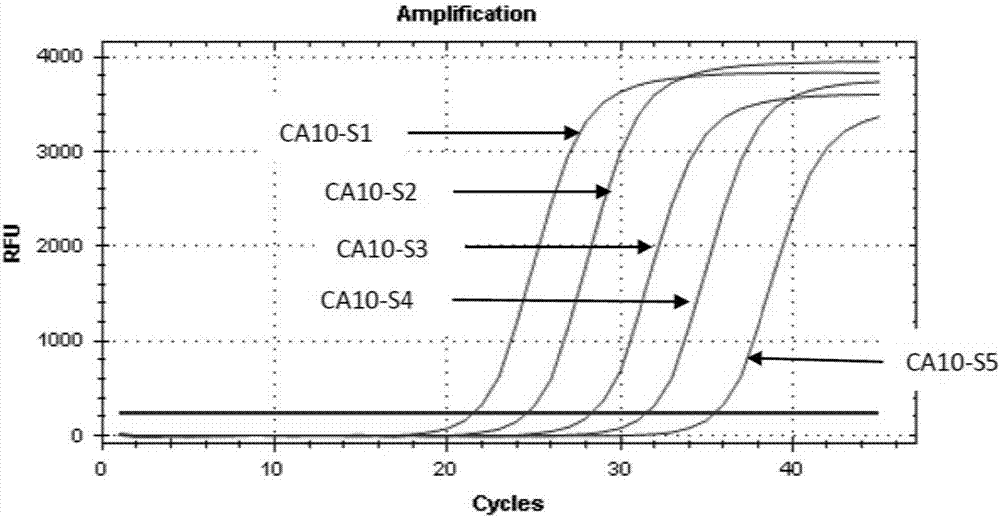
[0055] Example 2. Sensitivity analysis of coxsackievirus A6 and A10 type combined detection kit.
[0056] 21. Preparation of test samples:
[0057] Take 1×10 7 Coxsackievirus A6 plasmid (CA6-S1) of copies / ml was diluted 1:10, 1:100, 1:1000, 1:10000 to obtain CA6-S2 (1×10 6 copies / ml), CA6-S3 (1×10 5 copies / ml), CA6-S4 (1×10 4 copies / ml), CA6-S5 (1×10 3 copies / ml) were used as Coxsackievirus A6 detection samples.
[0058] Take 1×10 7 Coxsackie virus A10 plasmid (CA10-S1) in copies / ml was diluted 1:10, 1:100, 1:1000, 1:10000 to obtain CA10-S2 (1×10 6 copies / ml), CA10-S3 (1×10 5 copies / ml), CA10-S4 (1×10 4 copies / ml), CA10-S5 (1×10 3 copies / ml) were used as Coxsackievirus A10 detection samples.
[0059] 22. Preparation of reagents:
[0060] Take 20 μl of the mixed solution prepared in step 131 in Example 1 and place it in a thin-walled PCR tube or PCR reaction plate, and then put each test sample prepared in step 12, positive control substance, DEPC-H 2 O Add 5 μl eac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com