Preparation method of O-type foot-and-mouth disease virus-like particle antigen, prepared O-type foot-and-mouth disease virus-like particle antigen and application thereof
A foot-and-mouth disease virus and particle antigen technology, which is applied in the fields of molecular biology and virology, can solve the problems of large-scale epidemics of diseases, increase the difficulty of vaccines, and the virus is not completely killed or fully attenuated, and achieves the stability of foot-and-mouth disease virus-like particles. , The effect of soluble expression with high yield and no biosafety risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Expression of embodiment 1 recombinant FMDV capsid protein SUMO-VPO, SUMO-VP3 and SUMO-VP1
[0069] 1.1 Preparation and transformation of recombinant plasmids
[0070]The O-type foot-and-mouth disease virus VP0 gene fragment shown in the sequence listing SEQ ID NO.1, the O-type foot-and-mouth disease virus VP3 gene fragment shown in the sequence listing SEQ ID NO.2, and the sequence listing SEQ ID NO were synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd. . The O-type foot-and-mouth disease virus VP1 gene fragment shown in 3 is connected to the pETSUMO vector respectively. Then the fragments containing RBS-SUMO-VPO, T7-RBS-SUMO-VP3 and T7-RBS-SUMO-VP1 were amplified using the successfully ligated recombinant plasmids as templates. Step by step, the fragments obtained after digestion with corresponding endonucleases were cloned into the same pET28a expression vector to obtain a recombinant plasmid containing SUMO-VPO-SUMO-VP3-SUMO-VP1.
[0071] Transform the ligat...
Embodiment 2
[0078] The influence of the Ulp1 protease of embodiment 2 different enzymatic activity units on the assembly of capsid protein
[0079] 2.1 Assembly of virus particles under different protease addition conditions
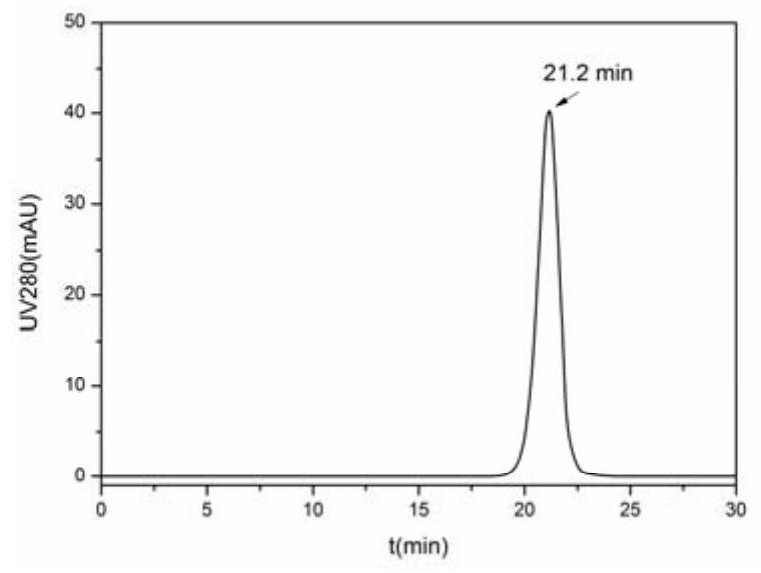
[0080] Dilute the purified protein solution containing recombinant FMDV capsid proteins SUMO-VP0, SUMO-VP3 and SUMO-VP1 to a total protein concentration of 1.0mg / mL, take 0.5ml of the above solution as a reaction system, and in five reaction systems 1U, 2U, 5U, 10U or 20U of Ulp1 protease were added respectively. Each protein sample was incubated at 4°C for 24 hours, and then nickel filler was added to adsorb SUMO protein. After treatment, the state of the protein in each reaction system was determined by high performance liquid gel filtration chromatography.
[0081] The result is as figure 2 as shown, figure 2 A-E show the results of high performance liquid gel filtration chromatography of the solution after Ulp1 protease was digested and adsorbed SUMO prote...
Embodiment 3
[0085] The impact of different salt concentrations of embodiment 3 on FMDV VLPs assembly
[0086] In this example, further investigation (NH 4 ) 2 SO 4 The effect of salt concentration on the assembly of FMDV VLPs. Specifically, unassembled recombinant FMDV capsid proteins SUMO-VPO, SUMO-VP3 and SUMO-VP1 were added to different concentrations (0.05M, 0.1M, 0.2M, 0.3M, 0.5M, 0.8M or 1.0M )(NH 4 ) 2 SO 4 Add 5U Ulp1 protease to 20mM disodium hydrogen phosphate (pH7.4) buffer solution, and incubate at 4°C for 24 hours, then add nickel filler to adsorb SUMO protein. After treatment, the high-performance liquid gel filtration chromatography was used to observe the influence of different salt ion concentrations on the assembly of FMDV VLPs.
[0087] The result is as Figure 4 As shown, the above experimental results under different salt ion concentration conditions correspond to Figure 4 A-G. Calculate the peak area, the results show that different salt ion concentrations...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com