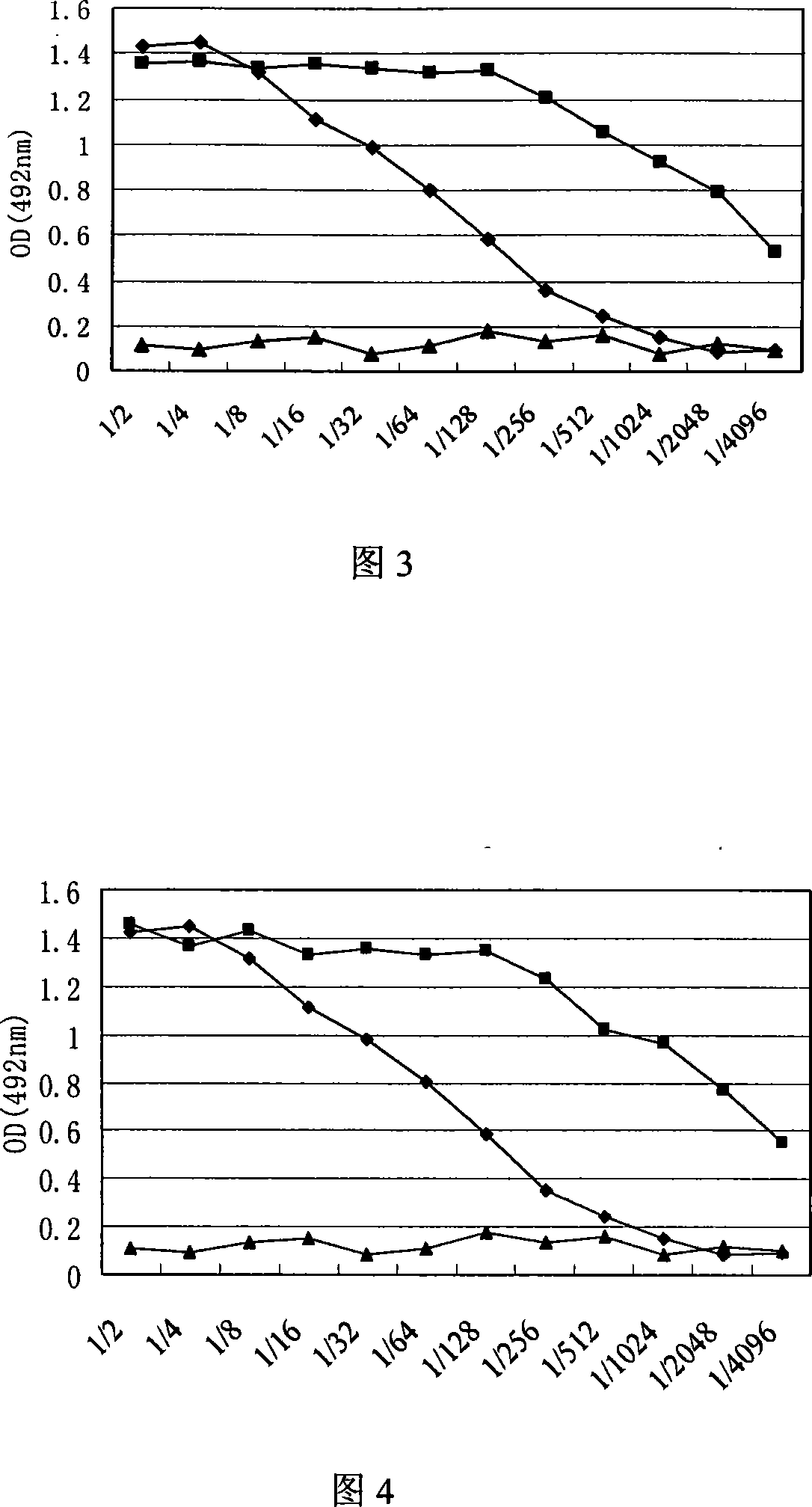
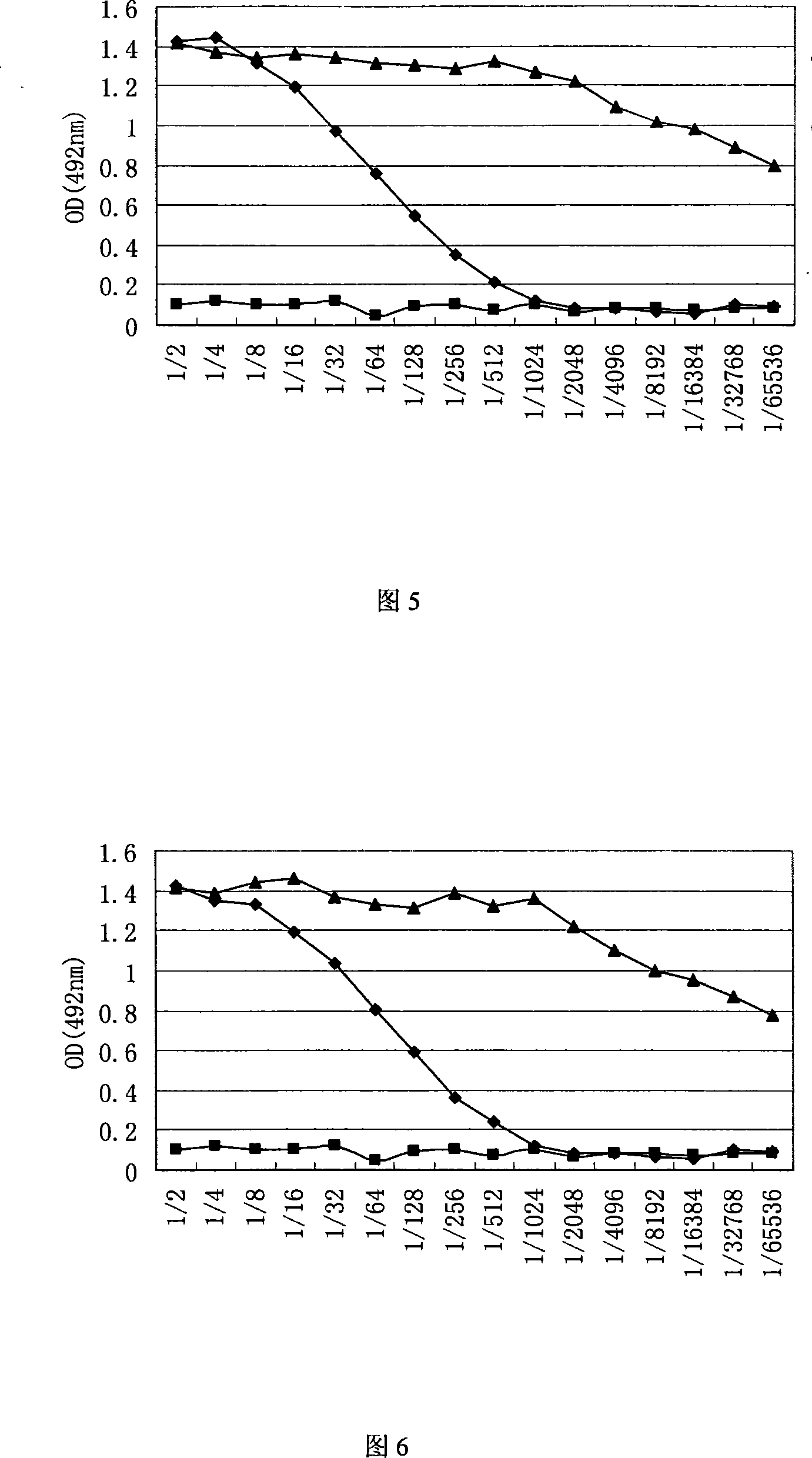
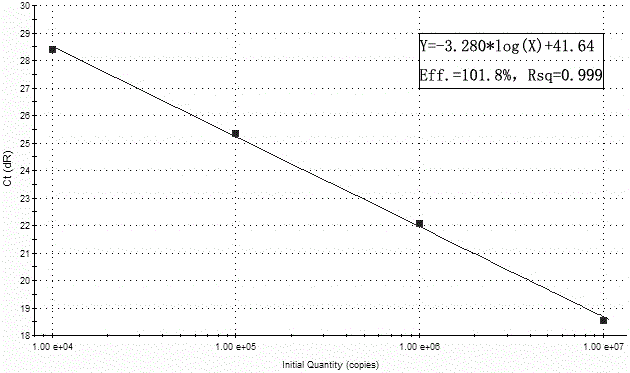
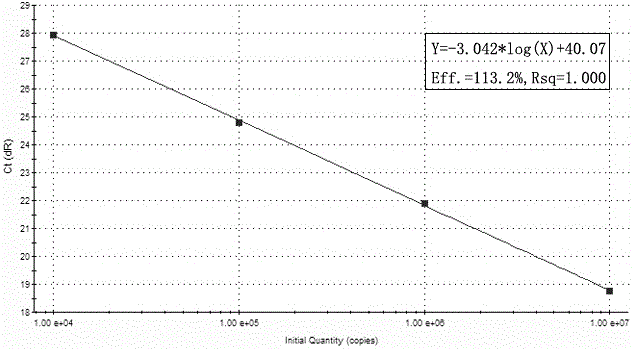
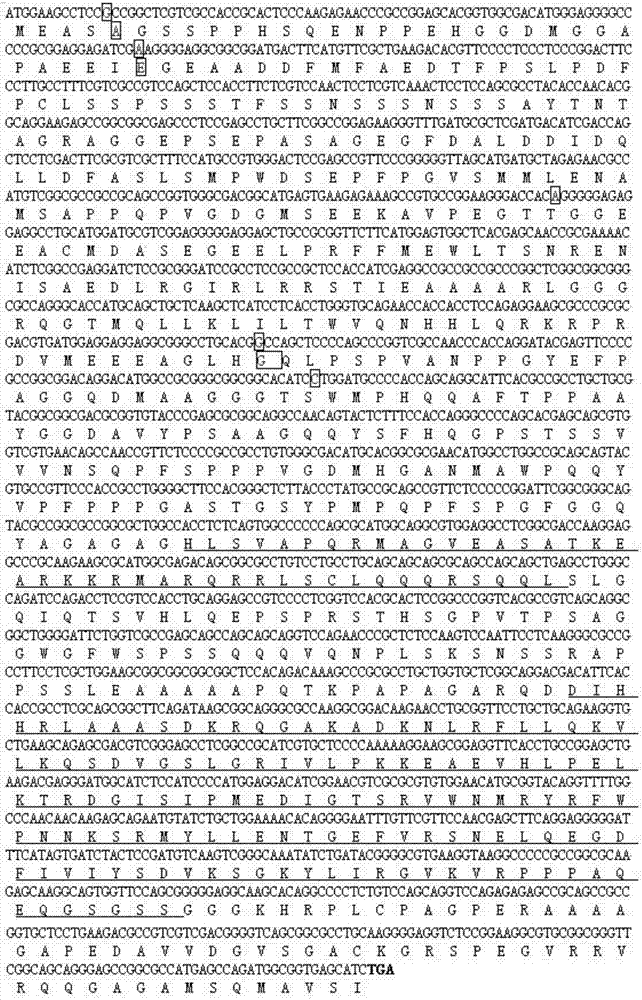
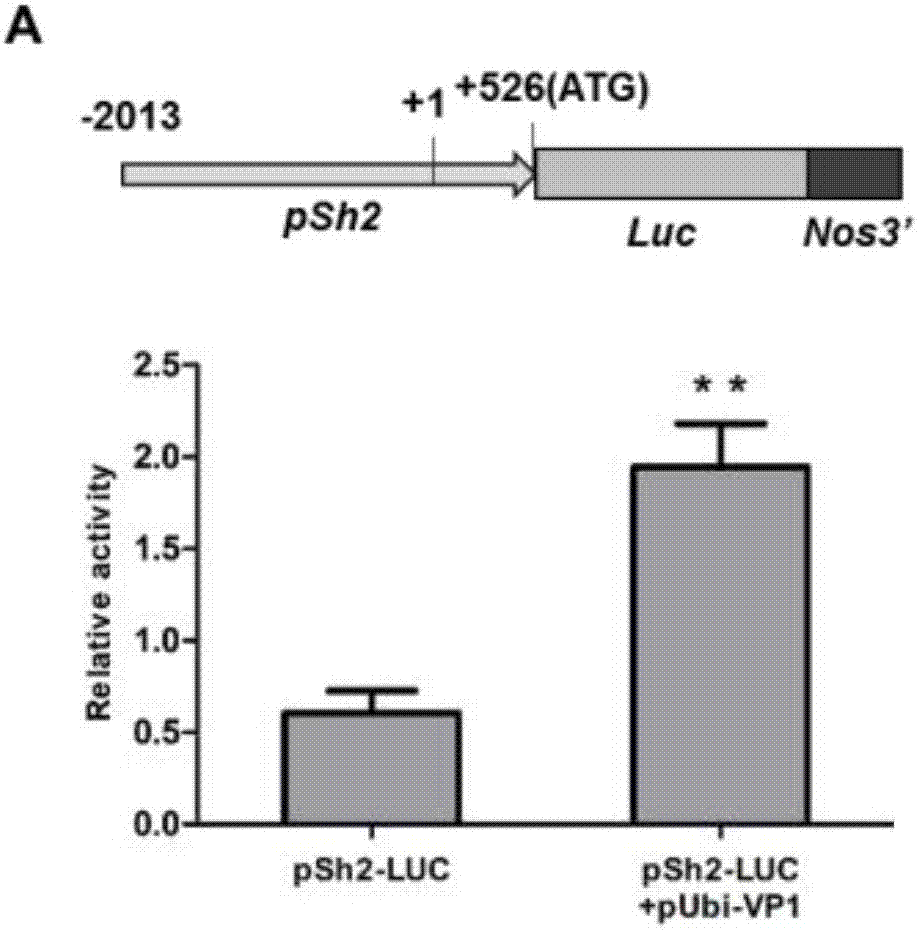
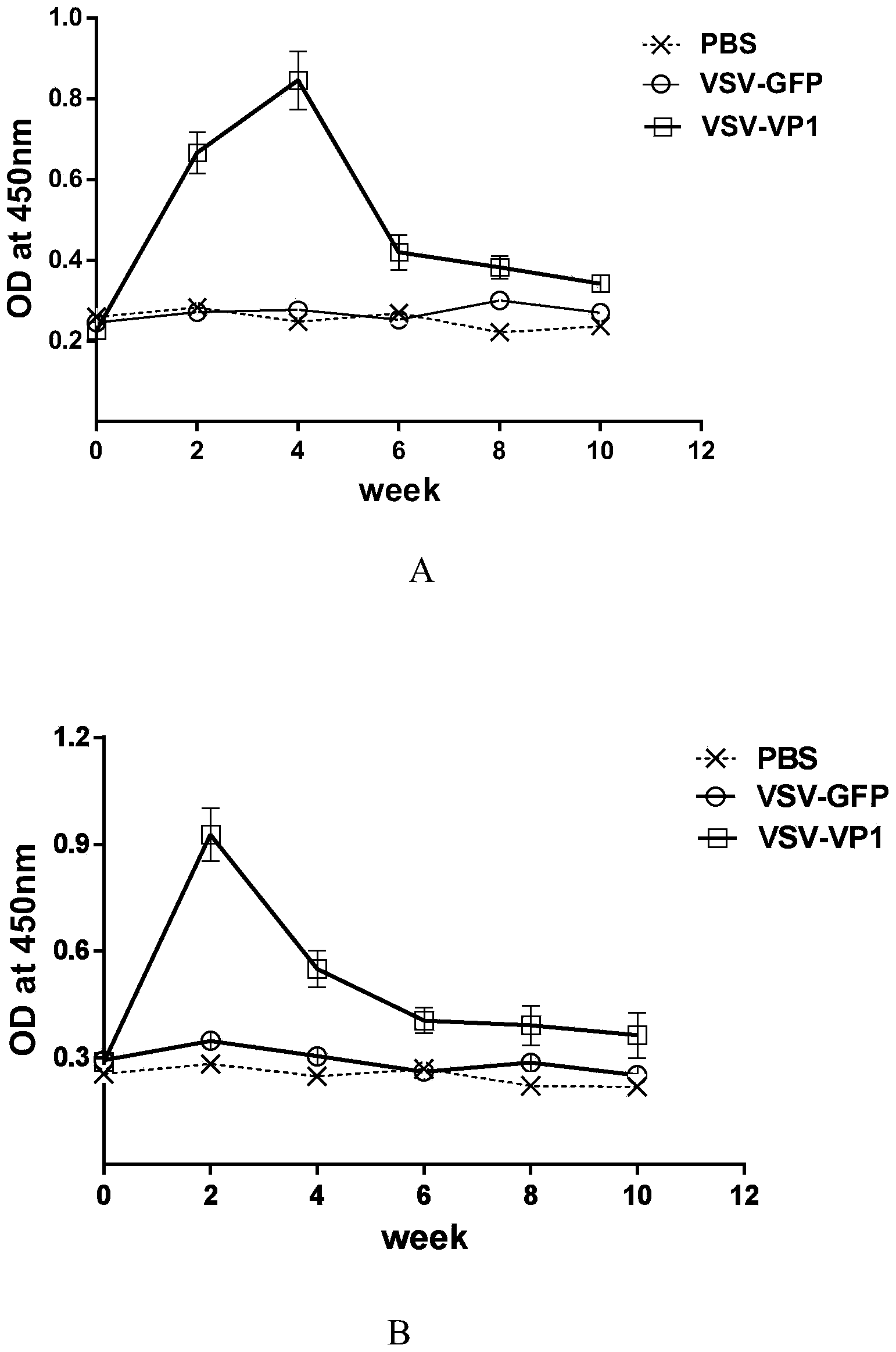
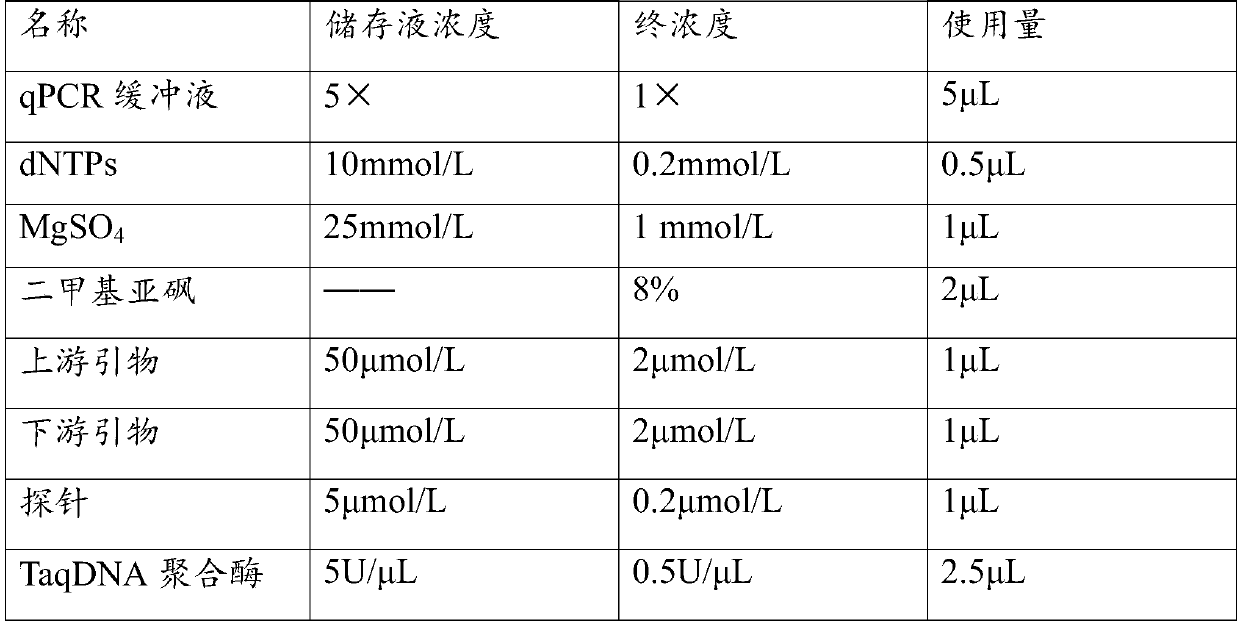
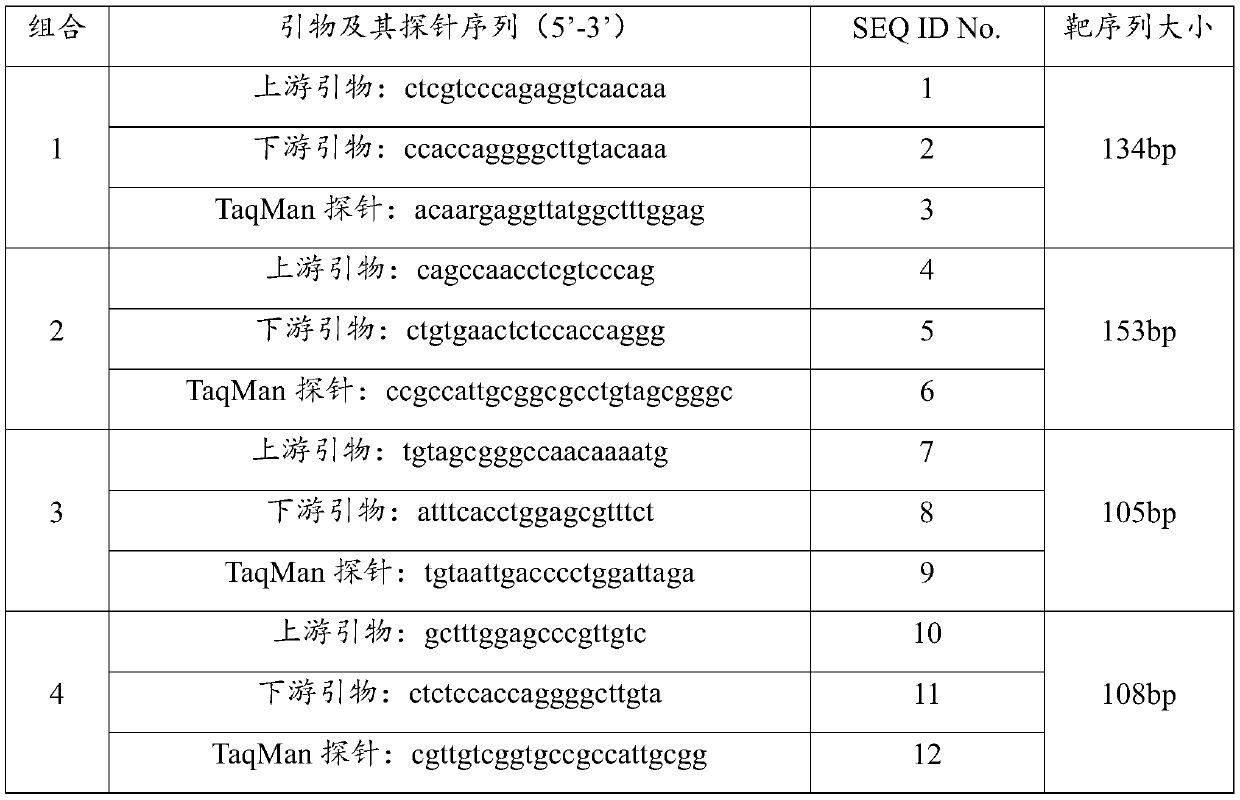
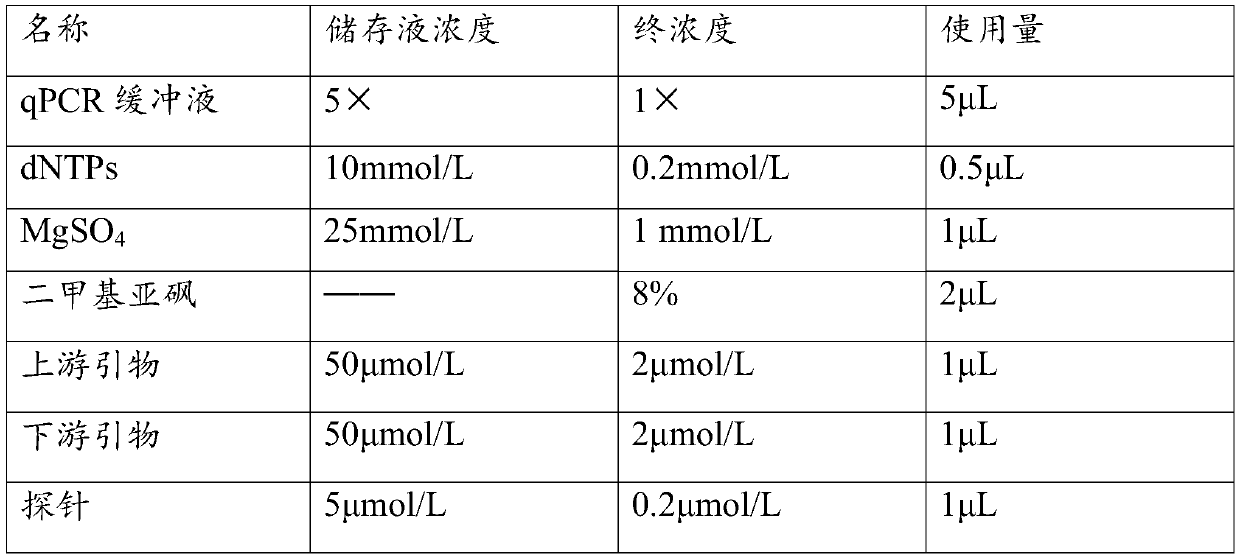
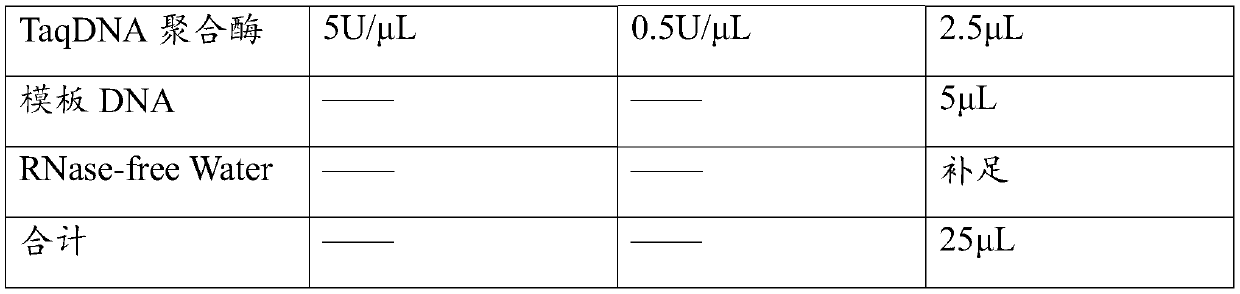
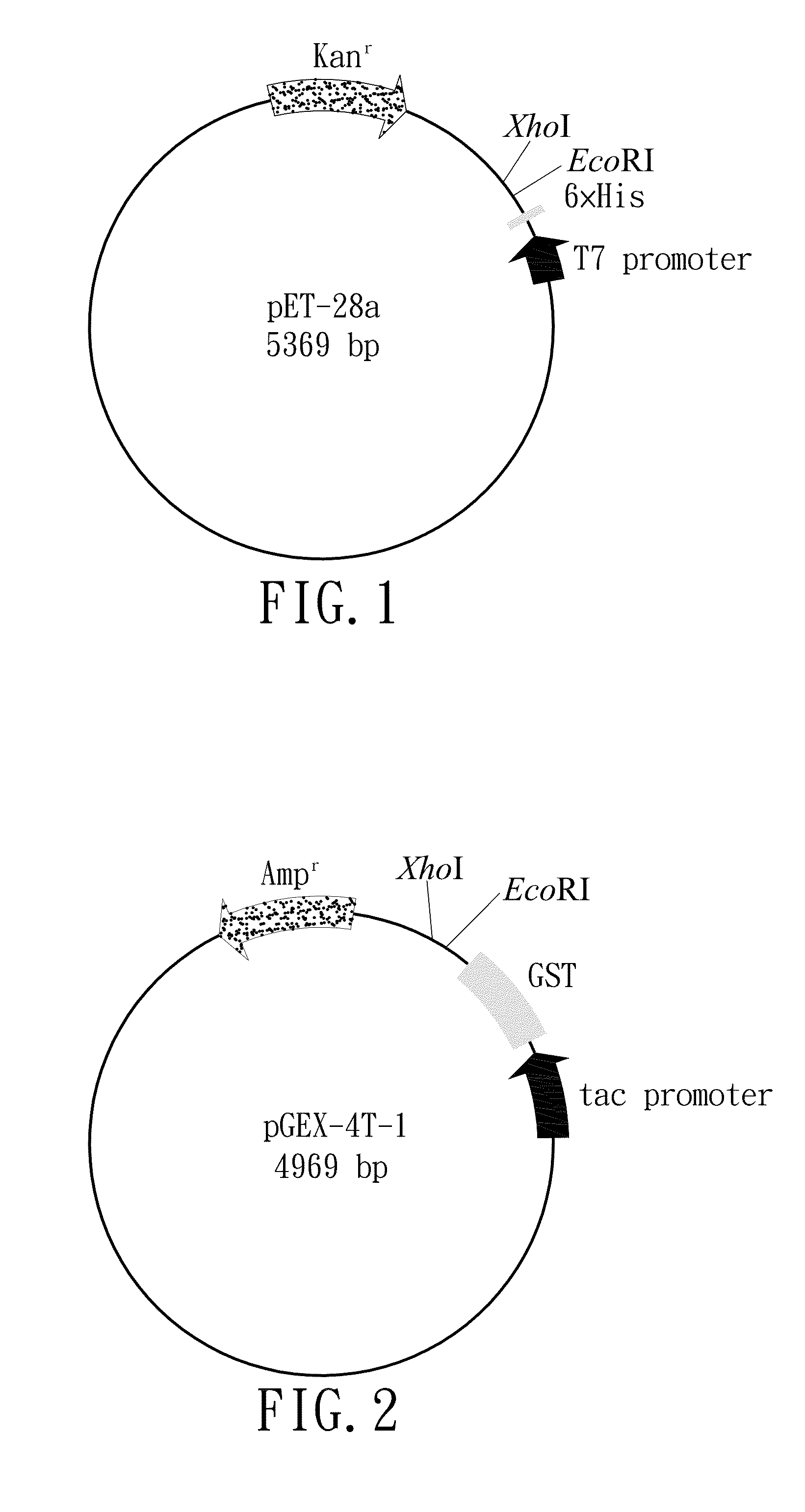
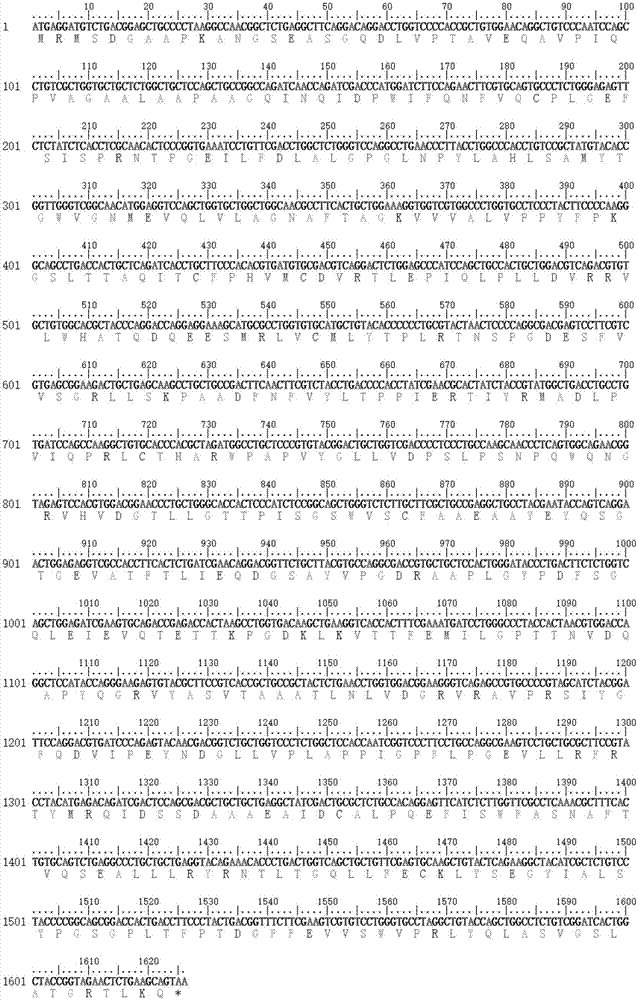
Patents
Literature
79 results about "Vp1 gene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Korean novel duck hepatitis viral antibody ELISA (Enzyme-Linked Immunosorbent Assay) detection kit
InactiveCN102127531ASolve the difficulty of purificationImprove securityRecombinant DNA-technologyFermentationDuck hepatitis A virusViral antibody
The invention discloses a Korean novel duck hepatitis viral antibody ELISA (Enzyme-Linked Immunosorbent Assay) detection kit. The detection kit contains an ELISA board coated by Korean novel duck hepatitis VP1 (Phenotypic Variance1) recombination protein, a sample diluent, concentrated washing liquid, an enzyme conjugate working solution, a chromogenic reagent (A), a chromogenic reagent (B), a stopping solution, a positive contrast solution and a negative contrast solution. The VP1 recombination protein is obtained by using the following method: using Korean novel duck hepatitis viruses as a material, augmenting and cloning the VP1 gene through an RT-PCR (Reverse Transcriptase-Polymerase Chain Reaction) method to obtain recombinant expression plasmid pMD (physical medium dependent)-VP1; then, directionally inserting to an expression vector pET-32a (+) and screening to obtain recombinant expression plasmid pET-32a(+)-VP1; and inducing, expressing and purifying by ITPG (Isopropyl beta-D-Thiogalactopyranoside) to obtain VP1 recombination protein. The detection kit is used for detecting the Korean novel duck hepatitis and has strong specificity, high sensitivity, simplicity of operation, easiness of popularization and application in a large-area range and wide market prospects.
Owner:POULTRY INST SHANDONG ACADEMY OF AGRI SCI
Method for preparing foot-and-mouth disease antigen
ActiveCN101121938APromote safe productionReduce consumptionSsRNA viruses positive-senseVirus peptidesAntigenTransfer vector
The invention provides a method for expressing foot-and-mouth disease antigens in insects using recombinant baculoviruses, which includes: cloning different gene combinations of foot-and-mouth disease into baculovirus delivery vectors to construct transfer vectors; using the constructed transfer vectors to transfer Infect the baculovirus and perform DNA recombination to obtain the recombinant baculovirus; infect the insect host with the recombinant baculovirus; culture the infected insect host to express the foot-and-mouth disease antigen; collect and purify the expressed foot-and-mouth disease antigen. The method of the present invention uses a baculovirus expression system to safely and efficiently produce foot-and-mouth disease antigens in a silkworm bioreactor. The prepared antigens are extremely safe and can directly produce vaccines to immunize animals. The method of preparing foot-and-mouth disease antigen of the present invention does not require investment in building a factory, has no three wastes, consumes very little energy such as electricity and water resources, and its production cost is also significantly lower than the traditional method of preparing foot-and-mouth disease antigen. It is safe, efficient, has low energy consumption and low cost. Low and many advantages.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI +1
Foot and mouth disease virus recombinant virus sample particle as well as preparation method and application thereof
The invention discloses a foot and mouth disease virus recombinant virus sample particle as well as a preparation method and application thereof. The recombinant virus sample particle is commonly assembled and expressed by components in a DNA molecule composition. The DNA molecule composition contains an O-type foot and mouth disease VP0 gene, an O-type foot and mouth disease VP1 gene and an O-type foot and mouth disease VP3 gene and further contains a green fluorescent protein gene. By virtue of the property that FMDV VLPs is self-assembled through VP0, VP1 and VP3, a construction method of a baculovirus recombinant vector is improved, a green fluorescent protein label is added into the vector, and FMDV VLPs is successfully prepared by virtue of a pFBDM Bac-to-Bac system, so that a theoretical foundation is laid for the further development of safe and efficient O-type FMDV genetic engineering vaccines.
Owner:CHINA ANIMAL HUSBANDRY IND
NDV (Newcastle disease virus) recombinant virus expressing DHAV-1 and DHAV-3 VP1 genes and application thereof
ActiveCN105754959AReduce manufacturing costAvoid interferenceSsRNA viruses negative-senseSsRNA viruses positive-senseDuck hepatitis A virusHepatitis A viruses
The invention belongs to the technical field of molecular biology and particularly relates to NDV (Newcastle disease virus) recombinant virus expressing DHAV-1 and DHAV-3 VP1 genes.The recombinant virus is recorded as rLS-1VP1-2A-3VP1 and is obtained inserting serially connected DHAV-1 and DHAV-3 VP1 genes into an NDV vector and carrying out saving.The NDV (Lasota strain) is used as the vector for the recombinant virus, the serially connected DHAV-1 and DHAV-3 VP1 genes are inserted into the NDV (Lasota strain) to obtain a vector, and the DNV recombinant virus co-expressing DHAV-1 and DHAV-3 VP1 genes is obtained by determining an optimal insertion site to insert the DHAV VIP1 gene; the recombinant virus is useful in preventing duck hepatitis A viruses (type 1 and type 3) and duck Newcastle disease and filling the current blank of DHAV-3 vaccines.
Owner:POULTRY INST SHANDONG ACADEMY OF AGRI SCI SHANDONG SPECIFIC PATHOGEN FREEN CHICKS RES CENT
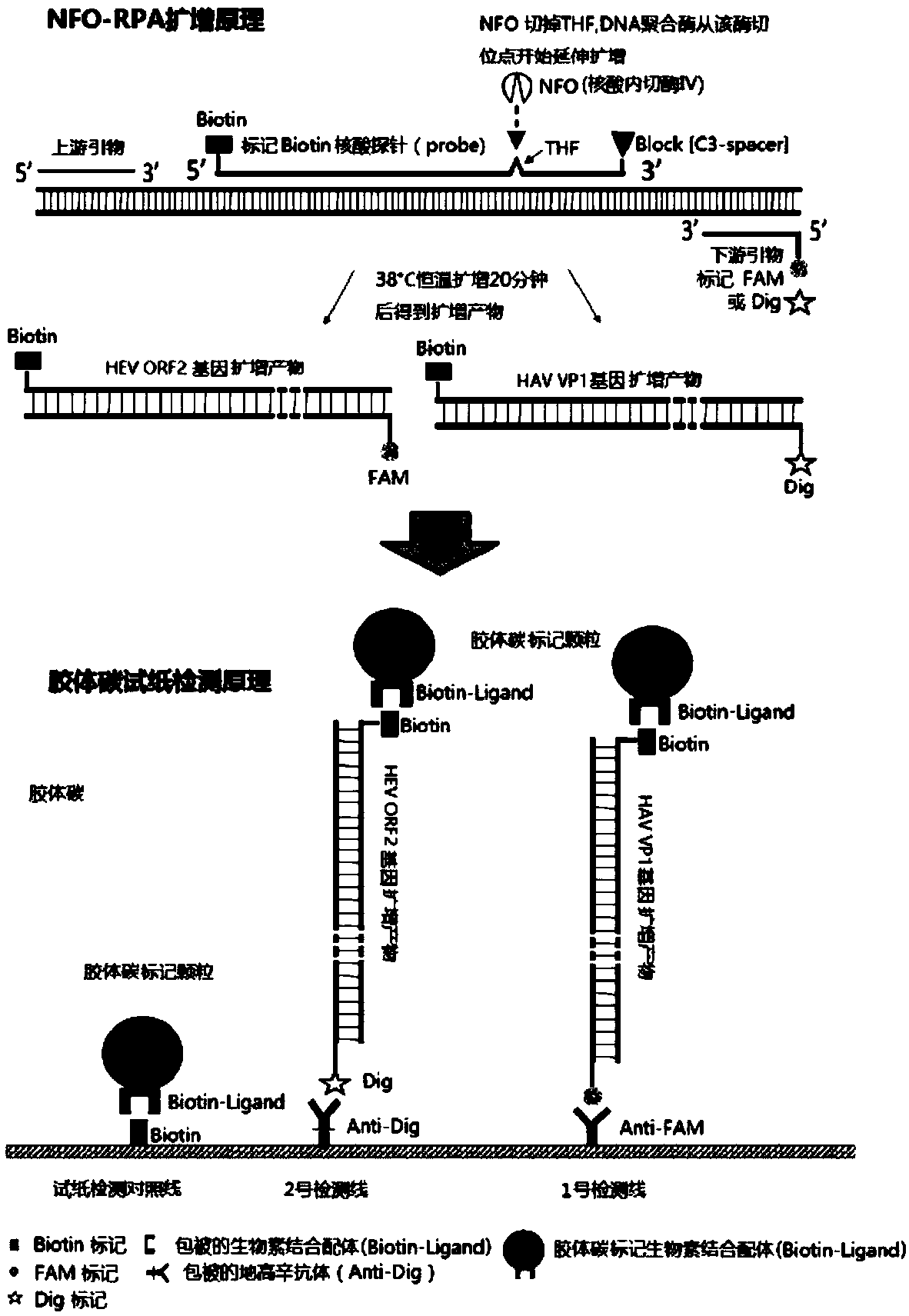
Primer for doubly detecting hepatitis e virus and hepatitis a virus through RT-RPA-lateral flow tomography, probe and kit
ActiveCN108841926AShorten detection timeThe reaction procedure is simpleMicrobiological testing/measurementAgainst vector-borne diseasesControl lineHepacivirus
The invention discloses a RT-RPA-lateral flow tomography kit for doubly detecting hepatitis e virus and hepatitis a virus. The kit comprises an upstream primer, an intermediate probe and a downstreamprimer which are applicable to hepatitis e virus ORF2 gene sequence in the RT-RAP amplification technology, and / or an upstream primer, an intermediate probe and a downstream primer which are applicable to hepatitis a virus VP1 gene sequence, general agents for recombinase polymerase amplification technology, and a lateral flow tomography test strip, wherein the lateral flow tomography test stripcomprises a sample adding pad, a control line, a #1 detecting line and / or a #2 detecting line; the control line, the detecting lines and the primers are combined with a probe label. With the adoptionof the kit, the hepatitis e virus ORF2 gene and / or hepatitis a virus VP1 gene in a sample can be rapidly, sensitively and specifically detected on site in a non-lab environment.
Owner:JINZHOU MEDICAL UNIV
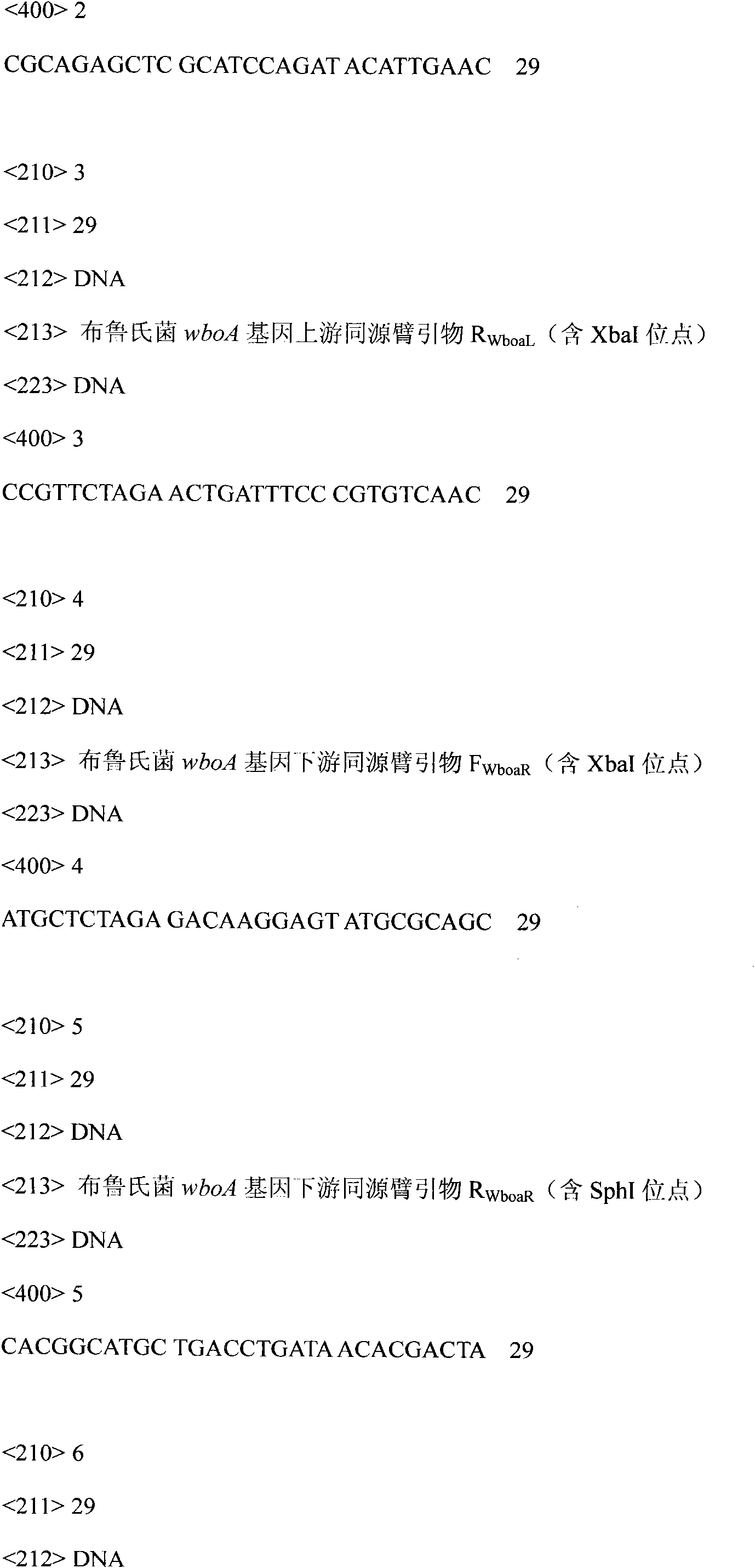
Recombinant brucella expressing VP1 gene of O-type foot-and-mouth disease virus and method for producing vaccines thereof
ActiveCN101979502AImprove securityStable genetic traitsAntibacterial agentsBacterial antigen ingredientsImmune effectsBrucella Vaccine
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Preparation method of engineered vaccine based on CSF-FMD duplex gene
InactiveCN103933581APrevent and Control InfectionReduce economic lossGenetic material ingredientsAntiviralsGenetic engineeringAnimal body
The invention relates to a preparation method of an engineered vaccine based on a CSF-FMD duplex gene, and discloses research and application of a duplex nucleic acid vaccine based on hog cholera virus-O type hog foot-and-mouth disease virus. Main materials of the duplex nucleic acid vaccine based on hog cholera virus-O type hog foot-and-mouth disease virus are as follows: pcDNA3.1-CSFV-E2-FMDV-O-VP1 eukaryotic plasmids. The preparation method disclosed by the invention researches nucleic acid vaccines for CSFVE2 gene and the FMDV-OVP1, can stimulate an animal body to generate corresponding high-level antibody and show very strong specificity. For the hog infection and propagation caused by CSFV and FMDV-O, the E2 gene and the VP1 gene achieve prevention and control effect on two viruses, so that safe and effective effects can be achieved.
Owner:GUIZHOU UNIV
Recombinant brucella for expressing Asia type-I foot and mouth disease virus VP1 genes and method for producing vaccines thereof
The invention relates to recombinant brucella for expressing Asia type-I foot and mouth disease virus VP1 genes and a method for producing vaccines thereof. Asia type-I foot and mouth disease virus (FMDV) Jiangsu strain VP1 genes are integrated into a brucella S2 strain genome, and the forming condition of an O chain in a smooth brucella cell wall LPS structure is simultaneously damaged so that the recombinant strain is changed into a rough type from a smooth type, the safety of the strain is further improved, but good immune effect on the brucella is still kept; and the recombinant strain is named as a recombinant brucella rS2-JS strain. The strain can express the Asia I FMDV VP1 protein, induces the generation of corresponding antibodies and has good basic immune effect on the Asia I FMD. The strain for producing the vaccines can change the current situation that brucella vaccine immunized animals and wild strain infected animals are difficult to distinguish, realizes cellular immunity of FMD vaccines at the same time, and provides a good vaccine for prevention and control of brucella diseases and FMD.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Primers, probes, kit and method for synchronous amplification detection of HCMV, HSV1, HSV2 and B19
InactiveCN106834542AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesWindow periodDisease rates
The invention relates to the technical field of biological detection, and particularly relates to primers, probes, a kit and a method for synchronous amplification detection of HCMV, HSV1, HSV2 and B19; the primers include four pairs of primers, namely totally 8 primers, for amplifying an HCMV virus UL75 gene, an HSV1 virus UL12 gene, an HSV2 virus UL15 gene and a B19 virus VP1 gene; the probes include 4 probes for detecting the HCMV virus UL75 gene, the HSV1 virus UL12 gene, the HSV2 virus UL15 gene and the B19 virus VP1 gene; the kit comprises the primers and the probes. The primers, probes, kit and method have the advantages of accuracy, sensitivity, short window period, high efficiency and the like, the operation is relatively simple, and the incidence rate of mother and infant vertical transmission diseases can be reduced.
Owner:东莞市儿童医院 +2
Application of transcription factor VP1 in regulating crop plant height and grain starch content
InactiveCN107058343AReduce plant heightHigh activityPlant peptidesFermentationCauliflower mosaic virusWild type
The invention discloses application of a maize transcription factor VP1 in regulating crop plant height and grain starch content. The maize transcription factor VP1 is inserted into a plant expression carrier carrying a plant selection gene, a target gene is started by a maize combined type Ubiquitin promoter, a selectable marking gene hptII is started by a 35S promoter from cauliflower mosaic virus; through genetic transformation of maize embryo callus mediated by agrobacteria, transgenic plants are obtained in batch. According to the maize transcription factor VP1 in regulating the crop plant height and grain starch content, the plant height and grain characters of homozygous strains of a transgenic offspring are authenticated, as is shown, compared with a wild type plant, the plant height of an overexpression VP1 gene plant is reduced by about 35%, and the starch content of seeds is increased by around 3%. A new path is opened up for increasing the height of plants of graminaceous crops like maize, sorghum, rice, and wheat, and increasing the content of grain starch of the graminaceous crops.
Owner:SICHUAN AGRI UNIV
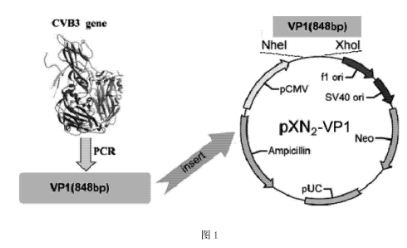
Viral myocarditis gene vaccine, its preparation method and application
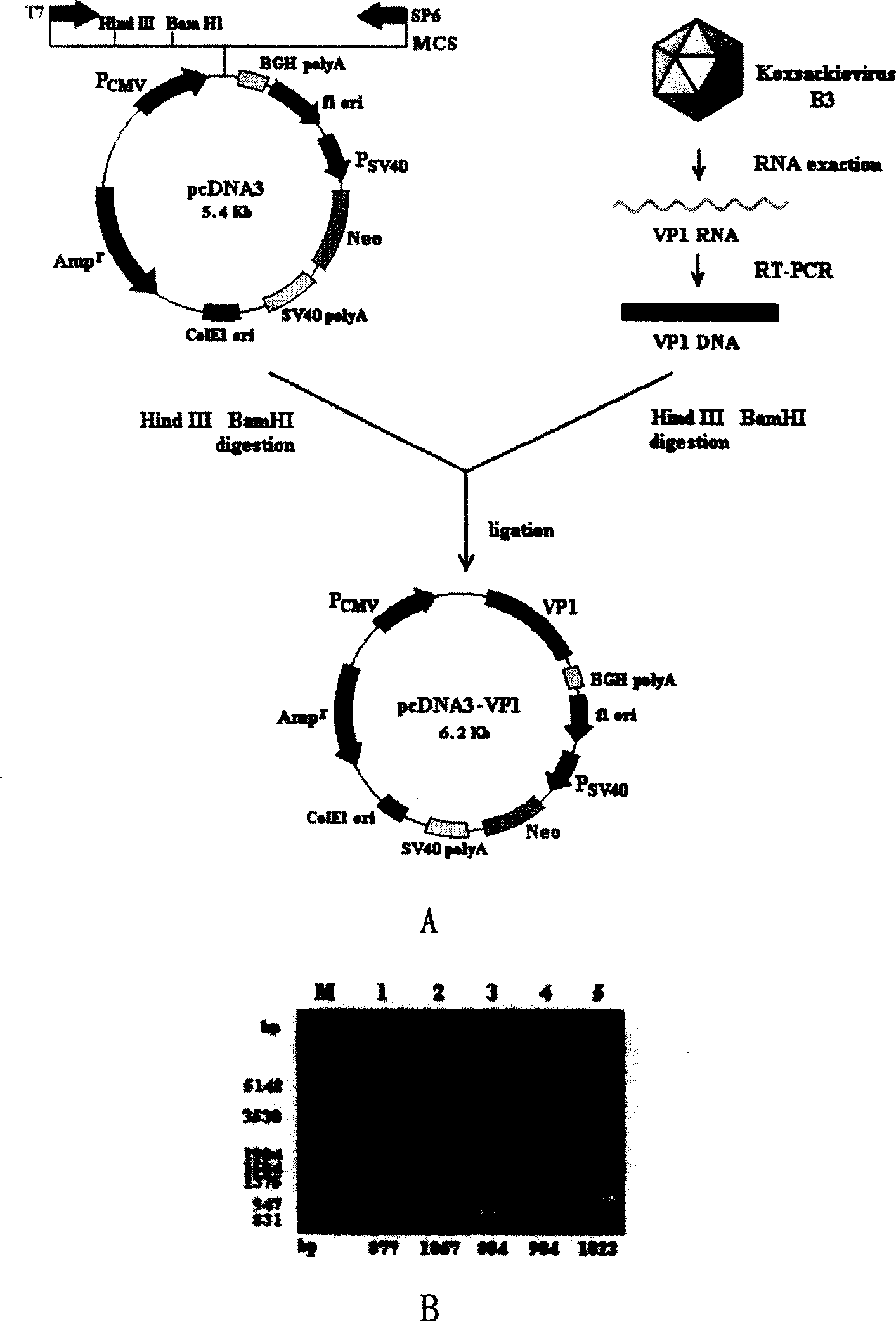
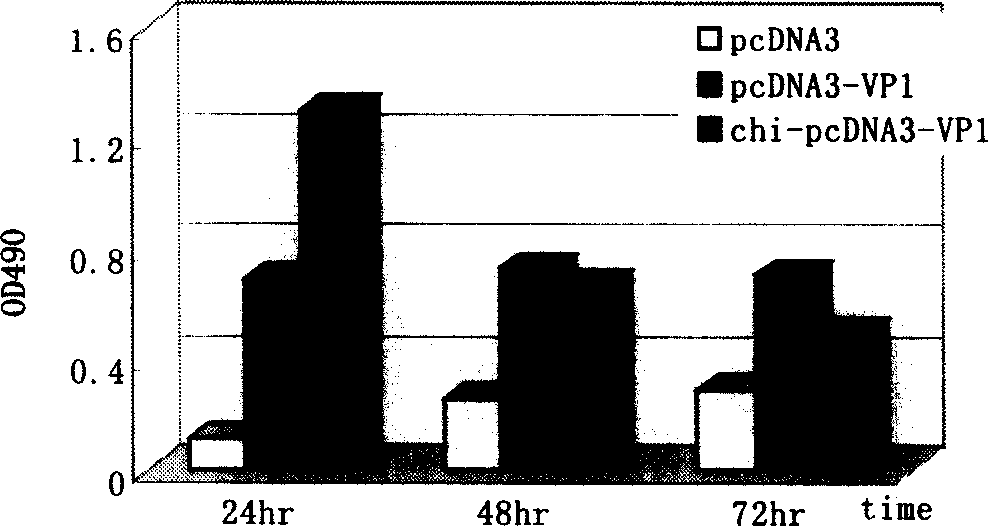
The present invention discloses a viral myocarditis gene vaccine, Said vaccine is constructed by using B3 type Coxsackie virus structural protein VPI gene and pcDNA carrier together, and its exterior is covered with a biological polysaccharide. It also discloses a method for preparing said gene vaccine and the application of said gene vaccine in preparation of medicine for preventing viral myocarditis.
Owner:上海欣安基因免疫与疫苗研究开发有限公司
Virus-like particle of senecavirus A and preparation method and application thereof
ActiveCN108642021AHigh expressionImprove assembly efficiencySsRNA viruses positive-senseVirus peptidesSolubilityProtein target
The invention discloses a virus-like particle of senecavirus A and a preparation method and application thereof. The virus-like particle of the senecavirus A is assembled by structural protein VP0, structural protein VP1 and structural protein VP3 of the senecavirus A, wherein the gene sequence of the encoding structural protein VP0 is shown in SEQ ID NO.1, the gene sequence of the encoding structural protein VP1 is shown in SEQ ID NO.2, and the gene sequence of the encoding structural protein VP3 is shown in SEQ ID NO.3. The method tries to combine different fusion tags to use so as to improve expression quantity of target proteins and assembling efficiency of the virus-like particle, and the result shows that after the N end of an SUMO VP1 gene combines with GST again, the solubility ofproteins expressed by expression bacterium which is transfected jointly by the combination of the obtained recombinant vector pGST / VP1, pSMK / VP0 and pSMC / VP3 is best, and the assembling efficiency ofVLPS is highest. The method provides technical support for further research and application of virus-like particle vaccines and accelerated transformation of animal vaccines from traditional inactivated vaccines to genetic engineering vaccines.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Codon-optimized EV71 VP1 gene and nucleic acid vaccine
InactiveCN101851631AHigh frequencyImprove expression levelGenetic material ingredientsAntiviralsEscherichia coliFhit gene
The invention belongs to the technical field of medicinal biology and discloses a codon-optimized EV71 VP1 gene and a nucleic acid vaccine. The EV71 VP1 gene has the use preference of a mammal cell and a colon bacillus codon and the sequence of the EV71 VP1 gene is SEQ IDNO.1. Based on the characteristic of the gene, the gene is inserted into a eukaryon expression vector so as to build the nucleic acid vaccine Vector / VP1, Vector / tPA-VP1 and Vector / VP1-tandem. All the three kinds of VP1 DNA vaccines can express VP1 protein better through eukaryotic cell expression and animal immune validation and can be stimulated to generate specific antibodies so as to embody high immunogenicity.
Owner:徐娟 +3
TaqMan probe-based Senecavirus A fluorescent quantitative RT-PCR (reverse transcription-polymerase chain reaction) detecting method and kit
InactiveCN109913591AStrong conservativeNo cross reactionMicrobiological testing/measurementDNA/RNA fragmentationSwine vesicular diseaseReverse transcriptase
The invention discloses a TaqMan probe-based Senecavirus A fluorescent quantitative RT-PCR detecting method and kit. The sequences of primers applied in the TaqMan probe-based Senecavirus A fluorescent quantitative RT-PCR detecting method are shown as SEQ ID NO.3 and SEQ ID NO.4, and the sequence of a probe applied in the TaqMan probe-based Senecavirus A fluorescent quantitative RT-PCR detecting method is shown as SEQ ID NO.5. The primers and the probe are obtained by performing sequence comparison and analysis according to the VP1 gene sequences of 41 SVA (Senecavirus A) strains epidemic in China since 2015 which are published in NCBI (National Center for Biotechnology Information) GenBank databases, and performing design within the conserved regions of the sequences; the sequences of theprimers and the probe are high in conservatism among analyzed virus strains. The TaqMan probe-based Senecavirus A fluorescent quantitative RT-PCR detecting method is high in specificity, sensitivityand stability, and can be successfully applied to clinical SVA inflection identification and diagnosis and provide good technical support for rapid swine vesicular disease identification and diagnosisin China.
Owner:HENAN ACAD OF AGRI SCI
Duck virus hepatitis suicide DNA vaccine, as well as constructing method and application thereof
InactiveCN102580116ASmall dose of immunizationAvoid hidden dangers such as immune toleranceGenetic material ingredientsAntiviralsDuck viral hepatitisTotal rna
The invention relates to the technical field of animal virology and animal infectious disease, and particularly relates to duck virus hepatitis suicide DNA vaccine, and the building method and the application thereof. The duck virus hepatitis suicide DNA vaccine provided by the utility model comprises DHV-VPI gene and alphavirus copying sub carrier pSCA 1, the constructing of the DNA vaccine comprises the following steps: the total RNA of DHV virus is extracted and purified, specific primers are designed, through the augmentation of RT-PCR, the total length cDNA gene order of DHV-VPI can be cloned, and finally, a purified VP 1 total length Cdna segment is restructured into the eukaryotic expression plasmid of alphavirus copying sub carrier pSCA 1. Compared with the prior art, the required immunizing dose of the vaccine provided by the invention is small, and the vaccine is safe and efficient; the vaccine can be eliminated by an organism with the Apoptosis of cells. Therefore, DNA plasmid are prevented from being possibly integrated into host cell chromosomes or causing hidden troubles such as immune tolerance and the like, and the duck virus hepatitis suicide DNA vaccine is efficient and stable, and the operation is convenient and quick.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Prokaryotic expression of enterovirus 71 type VP1 (virus protein 1) and vaccine containing VP1
InactiveCN102337289AEasy to operateLow costMicroorganism based processesAntiviralsEscherichia coliVirus Protein
The invention provides a prokaryotic expression method of an enterovirus 71 type VP1 (virus protein). The method comprises the steps of: cloning an enterovirus 71 type VP1 gene sequence into a prokaryotic expression carrier, transforming into coli bacillus to be expressed in an induction way, and purifying and renaturing the expressed VP1. The obtained VP1 and an immunologic adjuvant are matched with each other to prepare a vaccine. The invention has the advantages that: a genetic engineering recombinant VP1 is firstly expressed by a coli bacillus expression system, the operation is simple, the cost is low, large-scale production is easy to achieve and the like. The prokaryotic-expressed genetic engineering recombinant VP1 is matched with a chitosan adjuvant to immunize a mouse, and an immunological effect is evaluated, so that the VP1 is primarily proved to be not only capable of inducing the generation of a humoral immune protective reaction but also capable of inducing the generation of a cellular immunity reaction, therefore, the invention lays a foundation for further developing the genetic engineering recombinant VP1 and inactivated totivirus oral vaccine for human.
Owner:中国疾病预防控制中心病毒病预防控制所
Preparation method of soluble VP1 antigen of pig hidroa
The invention discloses a method for producing soluble VP1 antigen for detection of swine vesicular disease virus, which comprises the following steps of: 1). cloning a VP1 gene; 2). constructing rVP1; 3). Inducing the expression of rVP1 to generate soluble VP1; and 4). Purifying the soluble VP1. An inducer is IPTG with a concentration of between 0.05 to 1.00 mmol / mL; the induction temperature is between 16 and 20 DEG C. The method generates soluble VP1 protein in colon bacilli, reducing the production of inclusion bodies, avoiding complex processes of denaturation and renaturation. The obtained VP1 protein is high in purity, the same as SVDV particles in terms of immune trait and can be used as an antigen to replace SVDV particles to establish an indirect detection method for sensitive, specific, safe and reliable SVDV serum antibody.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Enterovirus 71 capsid protein 1 antigen, preparation method and immunodetection reagent
InactiveCN102242132AReduce riskAvoid contactMicroorganism based processesAntiviralsEscherichia coliAntigen
The invention discloses an escherichia coli optimal codon spliced enterovirus 71 (EV71) capsid protein 1 (VP1) antigen gene sequence. According to the invention, a reconstructed VP1 gene is obtained directly from 32 pieces of gene primer fragments through an annealing extension PCR (Polymerase Chain Reaction) technology, without an EV71 virus template. A reconstructed VP1 gene has a high expression level, can be purified to obtain a corresponding recombinant EV71-VP1 antigen. In addition, the method has higher security than a virus cracking method.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Recombinant virus for preventing viral myocarditis as well as vaccine and applications tof recombinant virus
ActiveCN103865890AActivate the immune systemConvenient inductionGenetic material ingredientsMicroorganism based processesWhole bodyPolymerase L
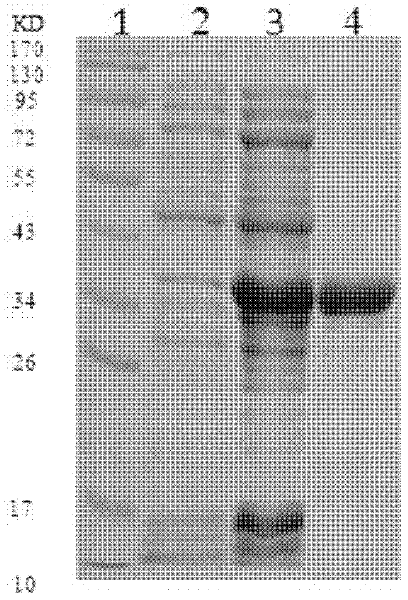
The invention discloses a recombinant virus. A vesicular stomatitis virus serves as a carrier, and an encoded antibody CVB3 structural protein VP1 gene is inserted between glycoprotein G and polymerase protein L of the vesicular stomatitis virus. The invention also provides a preparation method of the recombinant virus. According to the method, the recombinant virus is obtained by means of transfecting BHK21 cells via five plasmids: pP, pL, pXN2-VP1, pVSVG and pN. The invention also provides a vaccine which contains an effective dose of vaccine contains. A person can be vaccinated with the vaccine in a mucosa part by means of nasally dripping or orally taking, or through genital tracts, and the like. The technical problems is as follows: the conventional vaccine in the prior art can not effectively induce high-strength mucosa immune response, and the antibody can not effectively stay at a local mucosa part and consequently is insufficiently taken by APCs (Antigen Presenting Cells) are solved. The recombinant virus is capable of effectively enhancing CVB3 specific serum and local mucosa part antibody response, and remarkably strengthening the killing capability to local specific CD8T cells of whole-body and intestinal mucosa.
Owner:SUZHOU UNIV
Absolute fluorescent quantitative PCR detection method for chicken infectious anemia viruses
PendingCN112522447ARapid and specific detectionUnderstand the law of changeMicrobiological testing/measurementMicroorganism based processesSpecific detectionPcr method
The invention relates to an absolute fluorescent quantitative PCR detection method for chicken infectious anemia virus. Primers for specifically amplifying a VP1 gene segment of the chicken infectiousanemia virus are scientifically designed, and then the absolute fluorescent quantitative PCR method is established by using a TB Green<TM>Premix Ex Taq<TM>II reagent of the TaKaRa Company. Accordingto a highly conserved region of a VP1 gene of the chicken infectious anemia virus, upstream and downstream primers with the amplified fragment sizes of 140 bp are scientifically designed and synthesized, and the sequences of the primers are as follows: the sequence of the upstream primer is VP1-F: 5'-GCCCCGGTACGTATAGTGTG-3', and the sequence of the downstream primer is VP1-R: 5'-CCCGTACATGTGGTCTGCAT-3'. The method can be used for rapid and specific detection of chicken infectious anemia virus vaccine contamination residues and low-content chicken infectious anemia viruses in clinical samples by enterprises; and by quantitatively detecting the content of the chicken infectious anemia virus, the change rule and the like of the chicken infectious anemia virus in vivo can be known, and an effective means is provided for further researching the molecular biological characteristics of the chicken infectious anemia virus and making an effective prevention and control strategy.
Owner:YANGZHOU UNIV
Recombinant bifidobacterium for preparing EV (Enterovirus) 71 vaccine as well as preparation method and application of recombinant bifidobacterium
InactiveCN102703369AIncrease in vitro survivalPromote secretionBacteriaMicroorganism based processesEscherichia coliEnterovirus
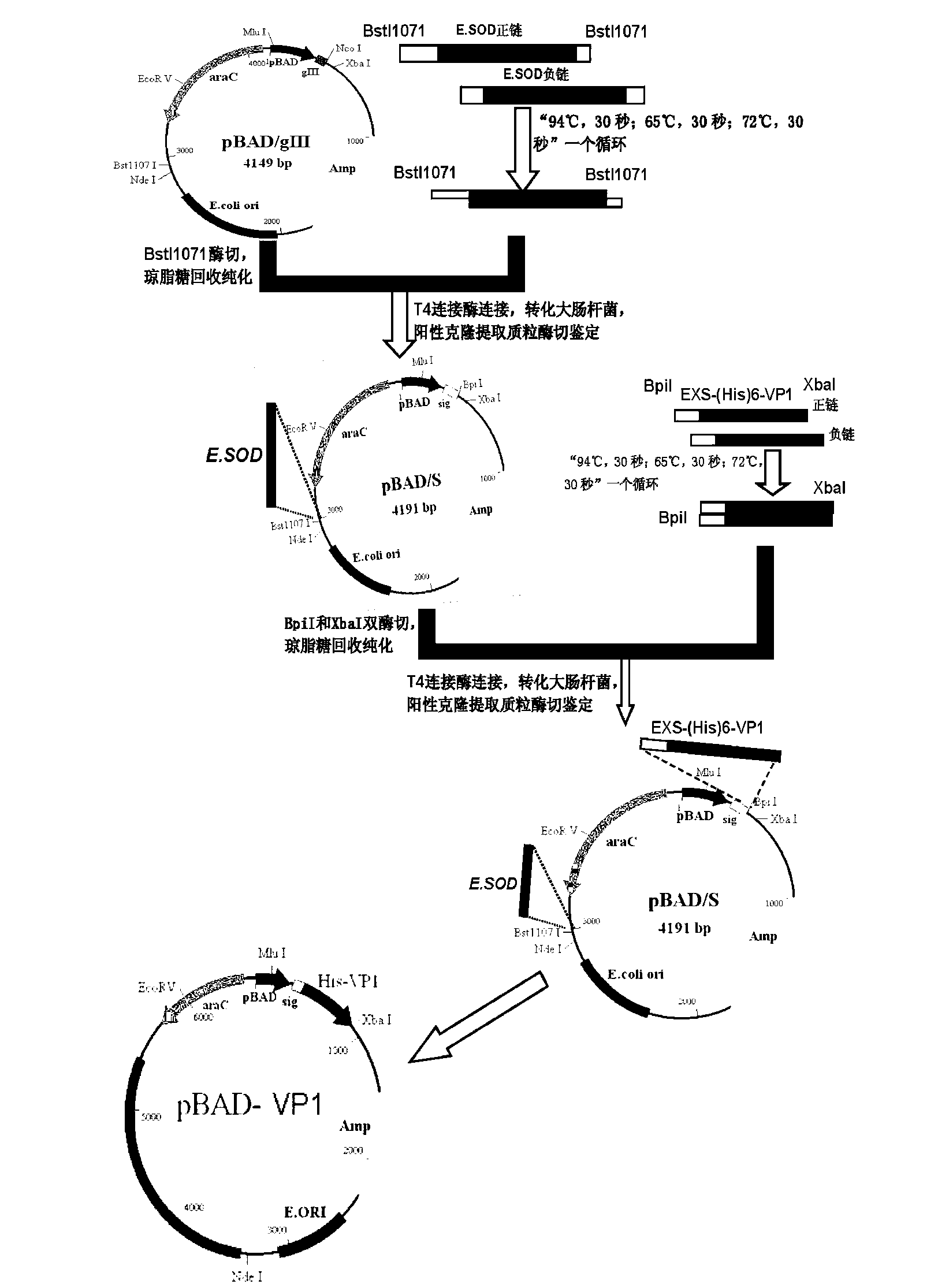
The invention discloses a recombinant bifidobacterium for preparing an EV (Enterovirus) 71 vaccine as well as a preparation method and application of the recombinant bifidobacterium and belongs to the technical field of genetic engineering. The recombinant bifidobacterium is transformed into a vector containing VP1 protein. The recombinant bifidobacterium is prepared by the following preparation steps of: (1) carrying out enzyme digestion on a recombinant double-stranded E.SOD (Escherichia coli. Superoxide Dismutase) sequence and pBAD (plasma Beam Assisted Deposition) / gIII vector and connection to obtain a pBAD / S vector; (2) inserting a double-stranded EXS-(His)6-VP1 gene into the pBAD vector subjected to double enzyme digestion and transforming Escherichia coli to obtain a positive clone pBAD-VP1 vector; and (3) transforming the pBAD-VP1 vector into bifidobacterium, screening pBAD-VP1 for transforming positive bifidobacterium to obtain the recombinant bifidobacterium for preparing the EV71 vaccine for treatment. The probiotic oral vaccine prepared by the recombinant bifidobacterium disclosed by the invention is free from injection administration and higher in economy and is a new mode for preventing the EV71 virus infection.
Owner:邓启文 +2
Method for detecting GI type Norovirus in seafood
InactiveCN109762944ANo cross reactionStrong specificityMicrobiological testing/measurementMicroorganism based processesMicrobiologyVp1 gene
The invention discloses a method for detecting GI type Norovirus in seafood. The method comprises the following steps of S1, taking the seafood as a sample to be detected, and extracting virus RNA; S2, adopting RNA or cDNA of the RNA as a template, and adopting a specific PCR primer composition for detecting the GI type Norovirus for performing PCR detection; S3, detecting the PCR product, and judging whether the seafood to be detected is contaminated with the GI type Norovirus, wherein the specific PCR primer composition of the GI type Norovirus includes a primer pair for specifically amplifying a GI type Norovirus VP1 gene, a sense primer of the primer pair is shown in SEQ ID No.1, and a reverse primer of the primer pair is shown in SEQ ID No.2. The primer composition has the advantagesof being good in specificity, high in sensitivity and coverage, and has the wide application prospect in the aspect of detecting the GI type Norovirus in seafood.
Owner:山东时进检测服务有限公司
Group-B type-III Coxsackie virus gene vaccine
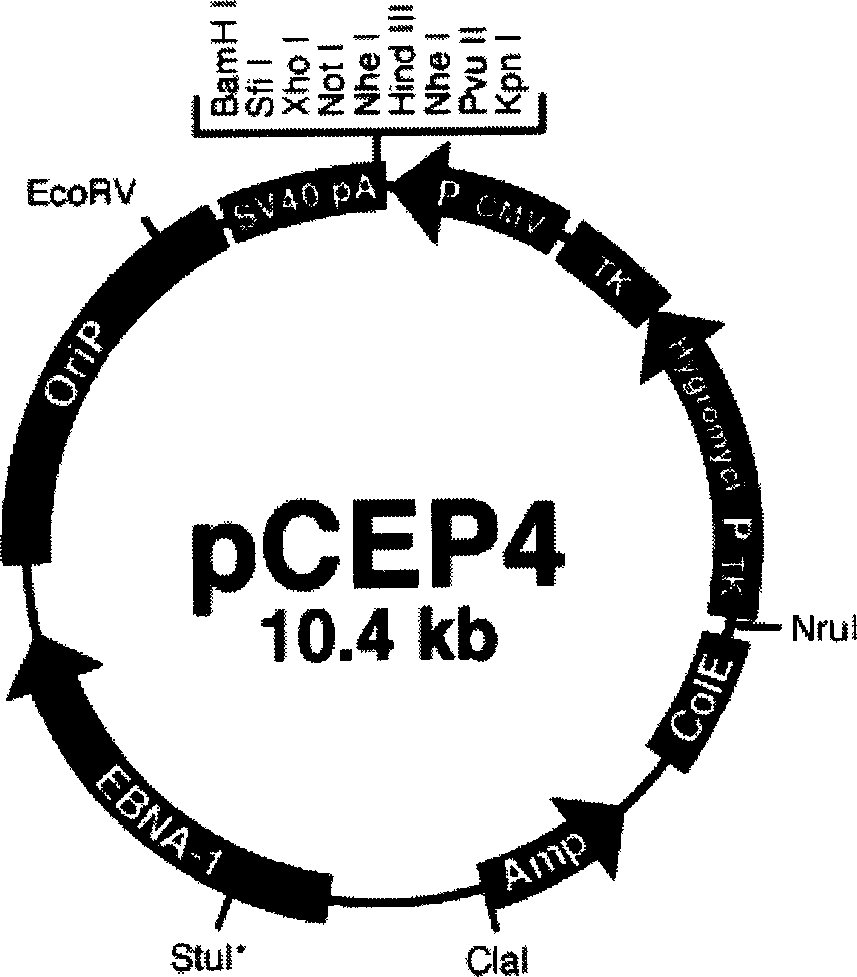
InactiveCN1772306AAvoid preparative purificationImmune response intactViral antigen ingredientsGenetic material ingredientsAntigen epitopeAntigen
The group-B type-III coxsackie virus gene vaccine is one kind of vaccine for preventing coxsackie virus infection. The present invention aims at providing CVB3 virus gene vaccine as one kind of pcCVP4-CVB1VP1 plasmid comprising VP1 gene with CVB3 coding main neutral antigen and plasmid pCEP4 as eukaryotic expression vector. Compared with traditional vaccines, the gene vaccine of the present invention has the following advantages: direct DNA inoculation without complicated antigen preparing and purifying process, integrated and lasting immune response with antigen polypeptide submission similar to that in natural infection and no antigen epitope altering, common physical and chemical characteristics with capability of embedding several destination genes in the identical plasmid to form combined vaccine, simple preparation process with low cost and high safety and stability for easy storing and transportation.
Owner:HARBIN MEDICAL UNIVERSITY
Codon optimization type 1 type duck hepatitis A virus VP1 gene and application of recombinant protein of codon optimization type 1 type duck hepatitis A virus VP1 gene
InactiveCN105063065AImprove performanceStrong specificityBiological testingGenetic engineeringDuck hepatitis A virusSerum ige
The invention discloses a codon optimization type 1 type duck hepatitis A virus VP1 gene and application of recombinant protein of the codon optimization type 1 type duck hepatitis A virus VP1 gene. A codon optimization type VP1 gene is obtained by optimizing a codon, a colloidal gold strip is prepared from recombinant protein of the codon optimization type VP1 gene and is used for detecting 1 type duck hepatitis A virus antibodies, and a colloidal gold strip which has higher performance and is used for detecting the DHAV-1 antibodies is obtained. The strip is high in sensitivity, and positive serum of DHAV-1 attenuated vaccine immunized ducks can be detected by the strip even after being diluted by 16-32 times. The strip has high specificity. When the 1 type duck hepatitis A virus antibodies are detected through the strip, high intra-assay and inter-assay repeatability is achieved. The prepared strip can be stored for several months under the temperature of 4 DEG C or under the room temperature of 25 DEG C.
Owner:SICHUAN AGRI UNIV
Preparation method and application of Coxsackie virus B3 virus-like particles
The invention discloses a preparation method of Coxsackie virus B3 virus-like particles. The method provided by the invention comprises the following steps: 1) cloning a VP1 gene and a VP4 gene of the Coxsackie virus B3 to a target plasmid 1 to obtain a recombinant expression vector 1; and cloning a VP2 gene and a VP3 gene to a target plasmid 2 to obtain a recombinant expression vector 2; 2) cotransforming the recombinant expression vector 1 and the recombinant expression vector 2 obtained in the step 1 ) to a yeast cell to obtain a recombinant yeast cell expressing the VP1 gene, the VP2 gene, the VP3 gene and the VP4 gene; and 3) cracking the recombinant yeast cell obtained in the step 2 ), and separating to obtain the Coxsackie virus B3 virus-like particles. Experiments show that Coxsackie virus B3 virus-like particles are successfully prepared by the method provided by the invention in the yeast expression system, and can be further used in candidate preventive vaccines and pharmaceutical compositions for virus myocarditis and diabetes type I.
Owner:BEIJING UNIV OF TECH
Method for detecting GII type norovirus in marine food
InactiveCN109868332ANo cross reactionStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationMicrobiologyVp1 gene
The invention discloses a method for detecting GII type norovirus in marine food. The method comprises the following steps: S1, taking marine food as a sample to be detected, and extracting virus RNA;S2, taking the RNA or cDNA of the RNA as a template, and carrying out PCR detection by using a specific PCR primer combination for detecting the GII type norovirus; S3, detecting the PCR product, andjudging whether the marine food to be detected is polluted by the GII type norovirus or not, wherein the specific PCR primer combination of the GII type norovirus comprises a primer pair for specifically amplifying a GII type norovirus VP1 gene, an upstream primer of the primer pair is shown as SEQ ID No.1, and a downstream primer of the primer pair is shown as SEQ ID No.2. The primer combinationused in the method disclosed by the invention has the advantages of good specificity, high sensitivity and high coverage degree, and has a wide application prospect in the aspect of detecting the GIItype norovirus in the marine food.
Owner:山东时进检测服务有限公司
Nucleic acid construct, recombinant vector, and recombinant e. coli producing chicken anemia virus vp1 protein
Disclosed herein is an expression cassette adapted to be expressed in an E. coli host cell and having a first nucleic acid fragment encoding a full-length chicken anemia virus (CAV) VP1 protein. In particular, the first nucleic acid fragment has a 5′-region that encodes a N-terminal amino acid sequence of the full-length CAV VP1 protein and is codon-optimized as compared to a corresponding 5′-region of a wild-type CAV vp1 gene, thus to encode the full-length VP1 protein. Specifically, the optimized codons are introduced into the corresponding 5′-region of the wild-type CAV vp1 gene by codon replacements.
Owner:CHINA MEDICAL UNIVERSITY(TW)
Construction of recombinant bacteria expressing avian encephalomyelitis virus VP1 protein
The invention relates to construction of a recombinant bacteria expressing avian encephalomyelitis virus VP1 protein. According to the invention, through synthesizing a primer of an avian encephalomyelitis virus VP1 gene, the recombinant bacteria with an efficient expression vector of the gene is constructed, efficient expression of the avian encephalomyelitis virus VP1 protein in Escherichia Coli is realized, and the amount of the expressed soluble protein accounts for about 60 percent of the total protein of the bacteria. The expression product is soluble protein in cytosol and has the antigen activity of natural VP1 protein, and avian encephalomyelitis VP1 subunit vaccine prepared from the expression product can effectively protect birds against the attack of avian encephalomyelitis virus. Therefore, a firm basis is established for industrially producing the avian encephalomyelitis subunit inactivated vaccine.
Owner:YEBIO BIOENG OF QINGDAO
Method for preparing pig sapporo virus antigen
The invention discloses a method for preparing a pig Sapporo virus antigen. The preparation method comprises the following steps: amplifying an entire capsid protein VP1 gene by taking RNA (Ribose Nucleic Acid) of an SaV CH430 plant as a template through an RT-PCR (Reverse Transcription-Polymerase Chain Reaction); cloning the entire capsid protein VP1 gene into a eukaryotic expression vector pFastBacTMHTB; transferring a positive plasmid constructed successfully into a competent cell containing a DH10 rhabdovirus skeleton; screening a cell of a recombinant rhabdovirus plasmid carrying the SaV VPI gene through three resistant culture media containing kanamycin, gentamicin and tetracycline; transfecting the obtained cell of the recombinant rhabdovirus plasmid carrying the SaV VPI gene with an sf9 cell to obtain a P1-generation rhabdovirus and a P2-generation rhabdovirus; infecting P2-generation virus liquid with the sf9 cell; harvesting a cell after 72 hours to obtain a pig Sapporo virus VP1 capsid protein and an assembled virus-like particle antigen (VLP), wherein the VLP is similar to a PoSaV natural virus in structure. By adopting the method, a PoSaV VP1 protein and a VLP antigen thereof can be obtained, and a basis is laid for the research of subunit vaccines.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Expression and purification of murine norovirus VP1 protein virus-like particles and preparation of polyclonal antibody
InactiveCN107988240AFully formedStable formationSsRNA viruses positive-senseSerum immunoglobulinsLaboratory mouseNew Zealand white rabbit
The invention discloses expression and purification of murine norovirus VP1 protein virus-like particles and preparation of a polyclonal antibody. According to the technical scheme, structural proteinVP1 genes of the murine norovirus are subjected to codon optimization for the first time, protein is expressed by utilizing a baculovirus expression system, the expressed protein forms the virus-likeparticles, and the antibody can simulate capsid protein of live viruses. For a VLPs antigen, the new Zealand white rabbit is immunized, the polyclonal antibody capable of resisting op.VP1 is prepared, the experiment proves that the polyclonal antibody can be used for Western Blot detection and IFA analysis, and a basic material is provided for detecting the MNV carrying condition of the laboratory mouse in a laboratory. Meanwhile, the VLPs antigen and the polyclonal antibody lay a foundation for further developing an ELISA detection kit.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com