Group-B type-III Coxsackie virus gene vaccine
A coxsackie virus and gene vaccine technology, applied in gene therapy, antiviral agents, virus antigen components, etc., can solve problems such as poor safety and poor immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
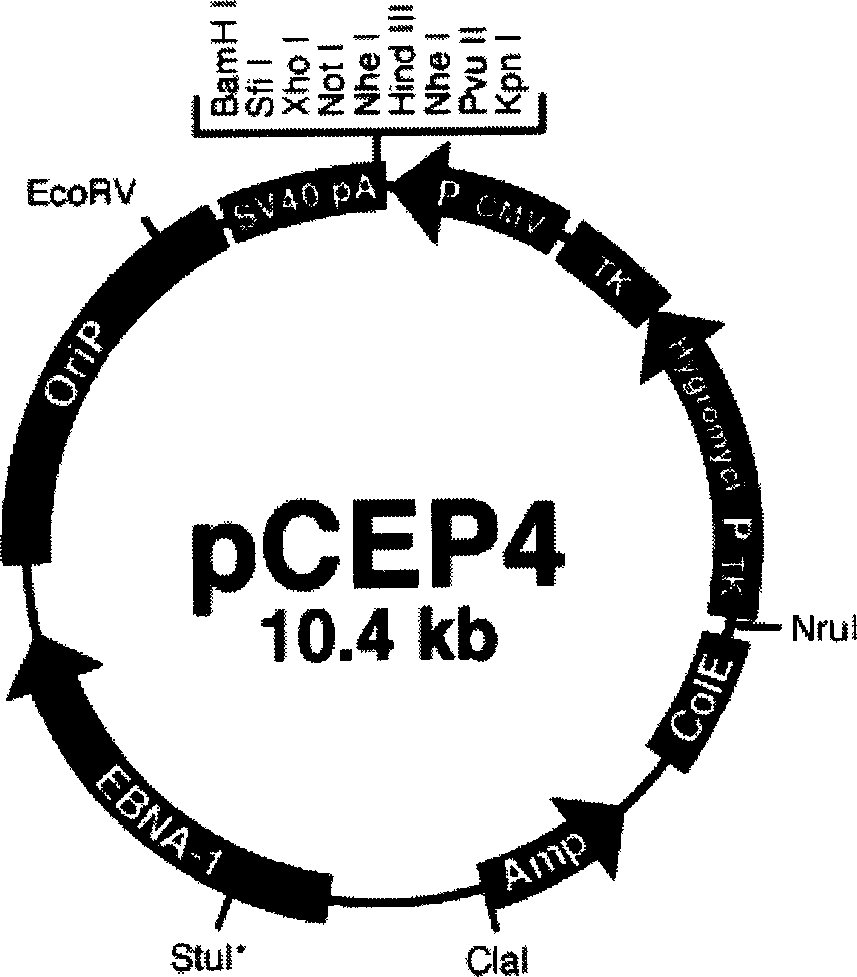
[0005] Embodiment 1: The CVB3 gene vaccine of this embodiment is a pCEP4-CVB3VP1 plasmid composed of the VP1 gene of CVB3 encoding the main neutralizing antigen and the plasmid pCEP4 as a eukaryotic expression vector. For the gene sequence of pCEP4-CVB3VP1, see the gene sequence below list.
specific Embodiment approach 2
[0006] Specific implementation mode 2: This implementation mode introduces the genetic vaccine of the present invention in detail.
[0007] 1. The materials used are:
[0008] 1. Virus: The CVB3 virus strain is a standard strain in my country, donated by Professor Zhong Xuekuan from the Keshan Disease Research Institute of the China Endemic Disease Center. The virus was passaged in Vero cells, and the TCID50 was 10-8.
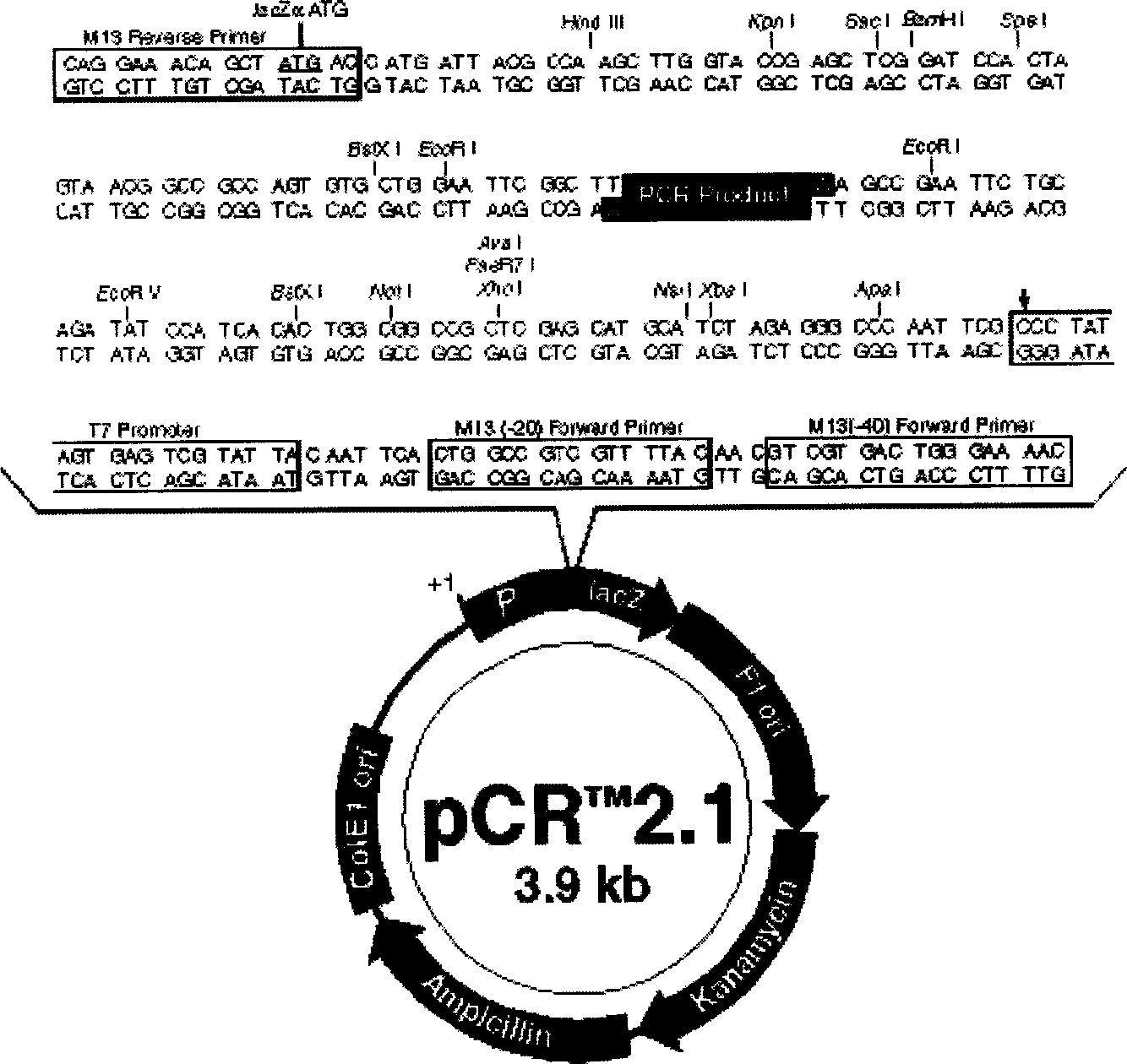
[0009] 2. Vectors and bacterial strains: The vectors used in the experiment include pCR2.1 and pCEP4, both of which are products of Invitrogen Corporation of the United States. The size of pCR2.1 is 3.9kb. It is a TA cloning vector, which is especially suitable for PCR product cloning. The vector contains Ptac promoter and LacZα gene, and the multiple cloning site is in the middle of the gene, so the recombinant can be preliminarily screened by blue-white selection . In addition, pCR2.1 has M13 forward primer and M13 reverse primer at both ends of the multip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com