Recombinant fusion protein and use thereof
A fusion protein and sequence technology, applied in the field of biomedicine, can solve the problems of disease progression, many adverse reactions, and unfixed course of treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Taking the HBcAg-N149 fragment as an example to prepare the hepatitis B core antigen protein carrier or its truncated fragment
[0061] The gene fragment encoding hepatitis B core antigen HBcAg-N149 can be obtained from hepatitis B virus particles (HBV) by PCR method, and 1-149 amino acid residues are intercepted, and B is added to the 5' and 3' ends respectively. wxya I and P st Restriction site for I. The HBcAg-N149 gene fragment passed B wxya I and P st Restriction digestion of I, ligation and introduction of Escherichia coli expression plasmid Pcold IV, recombinant Escherichia coli expression plasmid into Escherichia coli expression host strain JM109. Put the positively identified recombinant colonies into 5 mL LB culture medium, and culture with shaking at 37°C until the culture medium reaches A 600 Up to 0.4-0.6. Take 2 mL of the culture solution and add it to 200 mL of ampicillin-based LB culture solution, shake and culture for 3 h, then add IP...
Embodiment 2
[0062] Example 2 Insert B cell epitopes into reasonable sites of HBcAg-N149 to form fusion proteins as an example to prepare recombinant fusion proteins of HBcAg or truncated fragments carrying HBV therapeutic polypeptide epitopes
[0063] Insert the gene sequence encoding the 14th-24th amino acid sequence (DPRVRGLYFPA) derived from HBV Pre-S2 into the 78-79th amino acid of the HBcAg-N149 fragment by overlapping PCR, and add B to the 5' and 3' ends respectively wxya I and P st Restriction site for I. The HBcAg-N149 gene fragment passed B wxya I and P st Restriction digestion of I, ligation and introduction of Escherichia coli expression plasmid Pcold IV, recombinant Escherichia coli expression plasmid into Escherichia coli expression host strain JM109. Put the positively identified recombinant colonies into 5 mL LB culture medium, and culture with shaking at 37°C until the culture medium reaches A 600 Up to 0.4-0.6. Take 2 mL of the culture solution and add it to 200 ...
Embodiment 3
[0065] Taking recombinant HBcAg-N149 fragment and recombinant fusion protein HBcAg-N149 / preS2 as an example to illustrate the experiment of vaccine-induced HBV-specific immune response
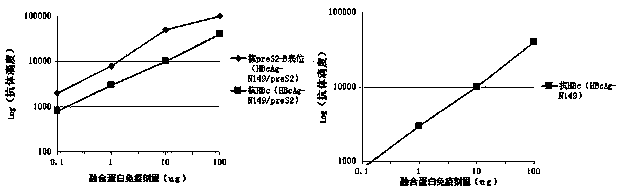
[0066] Three experimental groups were set up, namely the normal saline group, the HBcAg-N149 group, and the recombinant fusion protein HBcAg-N149 / preS2 group; tested in 6 female BALB / c mice (each group), each mouse was at 0, On days 21 and 42, 0.1-100 μg fusion protein and 10 μg aluminum salt adjuvant were subcutaneously injected.
[0067] One week after the second immunization, the antibody against the preS2-B epitope was detected, and the results were as follows image 3 shown, there are more than 10 5 Anti-preS2-B epitope antibody titer, and more than 10 3 The titer of the antibody against HBc showed a dose-dependent relationship; HBcAg-N149 could not induce antibodies against the preS2-B epitope; one week after the third immunization, the cellular immune response (IL4 and IFNγ) in the sp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com