Nanometer antibody for resisting hepatitis B virus core antigen and application of nanometer antibody
A technology of hepatitis B virus and nano-antibody, which is applied in the direction of antiviral agents, antiviral immunoglobulins, antitumor drugs, etc., can solve the problems of heavy economic burden, serious side effects, and long treatment cycle of patients, and achieve the reduction of HBeAg level, significant hepatitis B, significant therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
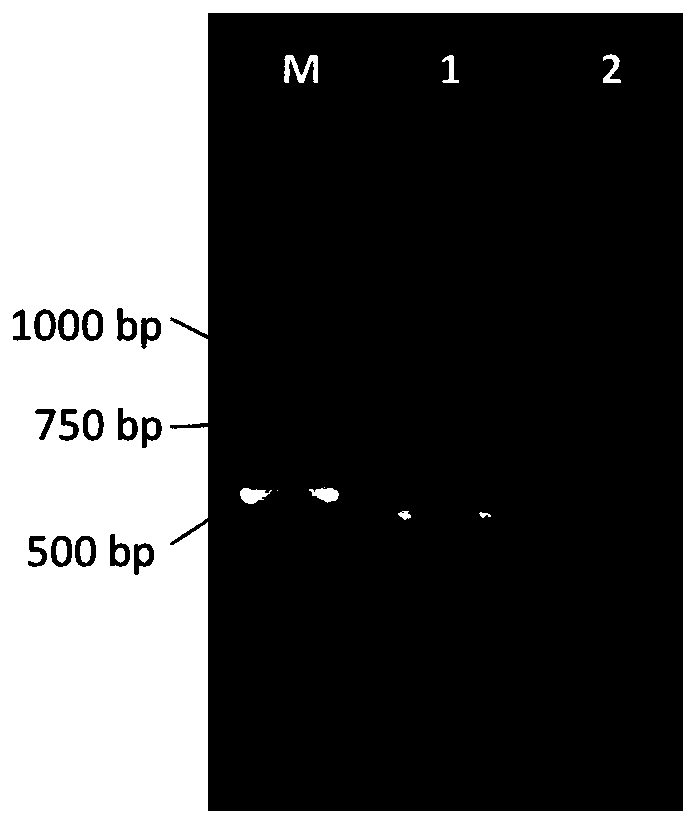
[0039] Embodiment 1: the preparation of HBcAg antigen
[0040] In this experiment, the immunogen used for alpaca is hepatitis B core antigen HBcAg, and the E.coli prokaryotic expression system is used, the bacteria are crushed and centrifuged, and the nickel column is used for separation and purification. Purified proteins were quantified using 12% polyacrylamide gels. Then the purified protein was subpackaged and freeze-dried and stored at -80°C (to avoid protein degradation caused by repeated freezing and thawing).
Embodiment 2
[0041] Example 2: Alpaca immunization and blood collection
[0042] We immunized the alpaca four times in total. The alpaca was immunized with HBcAg. The injection site was the cervical lymph nodes of the alpaca, and half of the samples were injected in the left and right lymph nodes of the alpaca neck. During the operation, attention should be paid to the whole process Preserve proteins and adjuvants at low temperature to avoid protein degradation caused by repeated freezing and thawing. The initial immunization dose is 200ug mixed with complete Freund's adjuvant, boosted with 100ug HBcAg mixed with incomplete Freund's adjuvant at the 2nd week, boosted with 100ug HBcAg mixed with incomplete Freund's adjuvant at the 10th week For booster immunization, mix 100ugHBcAg with incomplete Freund's adjuvant. Before each immunization, 10ml of blood was collected, anticoagulated with EDTA, and plasma and PBMC were separated.
[0043] After the fourth immunization, 40 ml of blood was t...
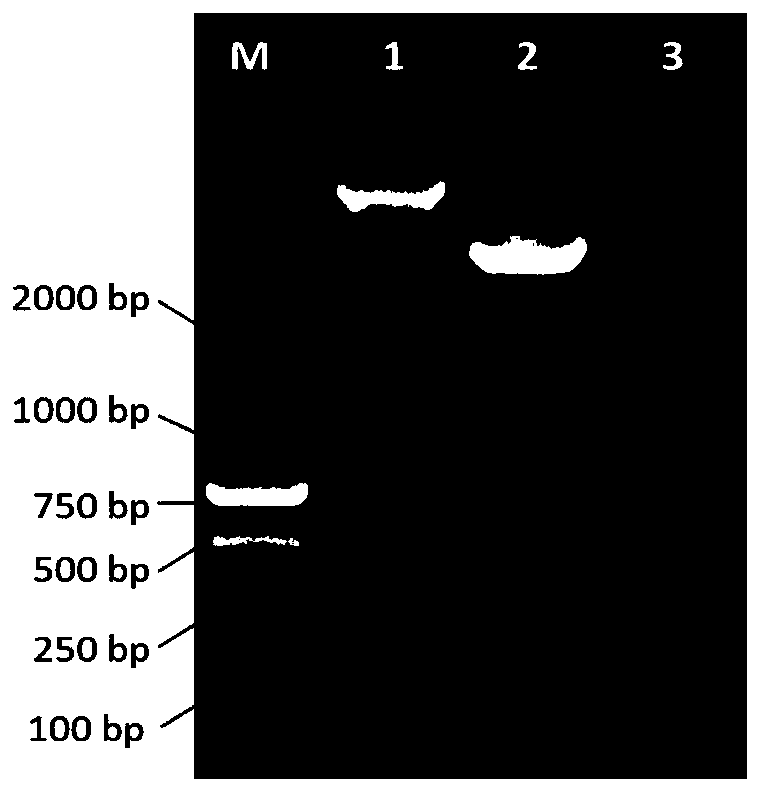
Embodiment 3
[0044] Example 3: Isolation of alpaca lymphocyte PBMC
[0045] Fresh blood samples should be stored at 18-20°C, and the separation of lymphocytes should be completed as soon as possible within 2-6 hours (preferably within 2 hours) after blood collection. The steps of lymphocyte separation are as follows: 1) Take out the Histopaque-1077 lymphocyte separation medium from the refrigerator at 4°C one day in advance, and place it at room temperature. 2) Take 5ml of Histopaque-1077 Lymphocyte Separation Medium at room temperature into a 15ml centrifuge tube, take 8ml of whole blood and add carefully and slowly above the liquid level of Histopaque-1077 Lymphocyte Separation Medium. 3) Centrifuge at 400g, 20°C, with a ramp-up rate of 0 and a ramp-down rate of 0, for 30 minutes. The centrifuged sample should be divided into four layers, which are serum, lymphocytes, dextran in lymphocyte separation medium and red blood cells from top to bottom. Carefully aspirate the monocytes in the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com