Dna-peptide combination vaccine
一种抗原肽、免疫应答的技术,应用在DNA / RNA疫苗接种、疫苗、逆转录DNA病毒等方向,能够解决疫苗有效性不能够充分满意等问题,达到减少次数的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0230] Elevation of antibody titers against angiotensin II caused by vaccine administration
[0231] (method)
[0232] Through PCR and ligation, a spacer sequence and angiotensin II amino acid sequence DRVYIHPF (SEQ ID NO: 21) were inserted between amino acid residues 80 and 81 of HBc, and a DNA fragment encoding modified HBc was obtained. The DNA fragment was subjected to TA cloning using pcDNA 3.1 / V5-His TOPO TA Expression Kit (Invitrogen) to obtain a pcDNA3.1-HBc-AngII vector.
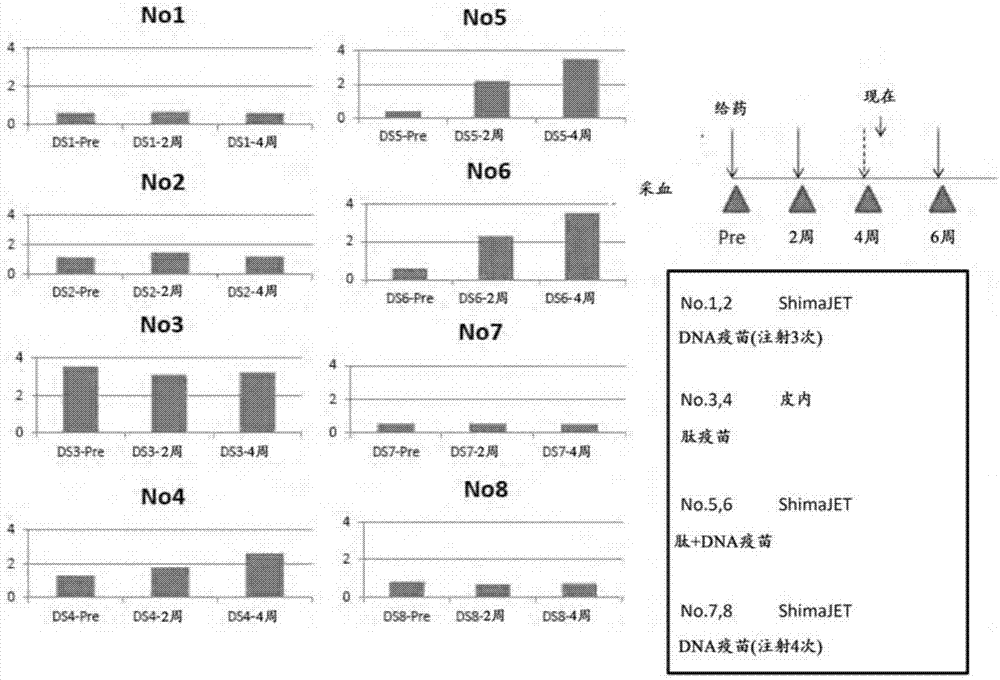
[0233] Divide 6 dogs into 3 groups (n=2 per group), use pcDNA 3.1-HBc-AngII to immunize according to the following 3 schemes, take the day of immunization as the 0th day, on the 0th day, 4 weeks later And 6 weeks later, the antibody titer against angiotensin II in peripheral blood was measured.
[0234] (I) Using a needleless syringe ShimaJET (trade name, Shimadzu Corporation), pcDNA 3.1-HBc-AngII prepared at 100 μg / 100 μl per administration was intradermally administered to dogs at four sites. ...
Embodiment 1
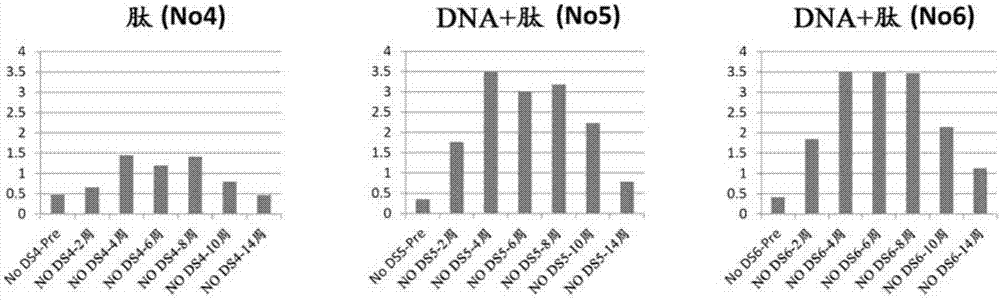
[0242] Increase of Antibody Titers Caused by DNA+Peptide Combination Vaccine
[0243] Divide 8 dogs into 4 groups (n=2 per group), use pcDNA 3.1-HBc-AngII and the conjugate of partial peptide of angiotensin II and KLH (AngII-KLH), according to the following 4 schemes For immunization, the antibody titer against angiotensin II in peripheral blood was measured over time.
[0244] (I) Using ShimaJET, 250 μg / 100 μl of pcDNA3.1-HBc-AngII and CpG DNA were prepared for each co-administration (total dose of 40 μg / time / animal), and intradermally administered to dogs at 4 sites . The dosage of pcDNA 3.1-HBc-AngII was 1.0mg / time / animal. This administration was performed three times on the 0th day, the 14th day, and the 42nd day. (DNA alone administration group 1)
[0245] (II) 12.5 μg / 250 μl of AngII-KLH and CpG DNA were prepared for each co-administration (total dose of 40 μg / time / animal), and intradermally administered to dogs at two sites. The dosage of AngII-KLH was 25 μg / time...
Embodiment 2
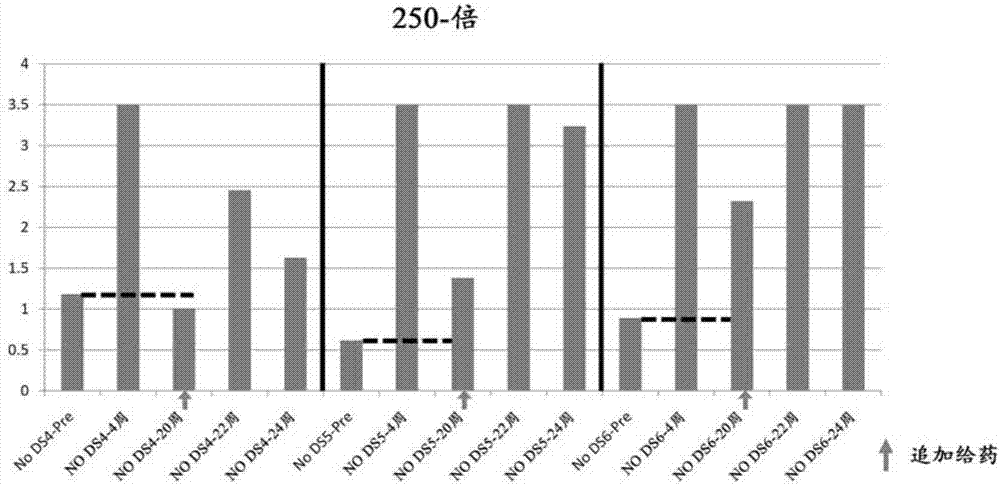
[0255] Effect of DNA+peptide combination vaccine on canine heart failure model
[0256] (method)
[0257] The effect of the DNA+peptide combination vaccine was studied using the following protocol.
[0258] · Dog: n=3
[0259] · Heart failure model: 4 weeks before the start of vaccine administration, mitral valve insufficiency was caused by chordotomy of the mitral valve, and a canine heart failure model was established.
[0260] Vaccine: pcDNA 3.1-HBc-AngII+AngII-KLH
[0261] ·Dosing schedule
[0262] (I) Vaccine administration group
[0263] [(pcDNA 3.1-HBc-AngII final concentration 250μg / 100μl+AngII-KLH final concentration 6.25μg / 100μl)×4 sites (DNA 1mg+peptide 25μg)+CpG (dosage 40μg / time / body)]×3 times (Day 0, Day 14 and Day 42) (ShimaJET)
[0264] (II) Control group
[0265] Normal saline × 4 sites × 3 times (Day 0, Day 14, and Day 42) (ShimaJET)
[0266] ·Evaluation items
[0267] Time-dependent changes in antibody titers against angiotensin II in peripheral b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com