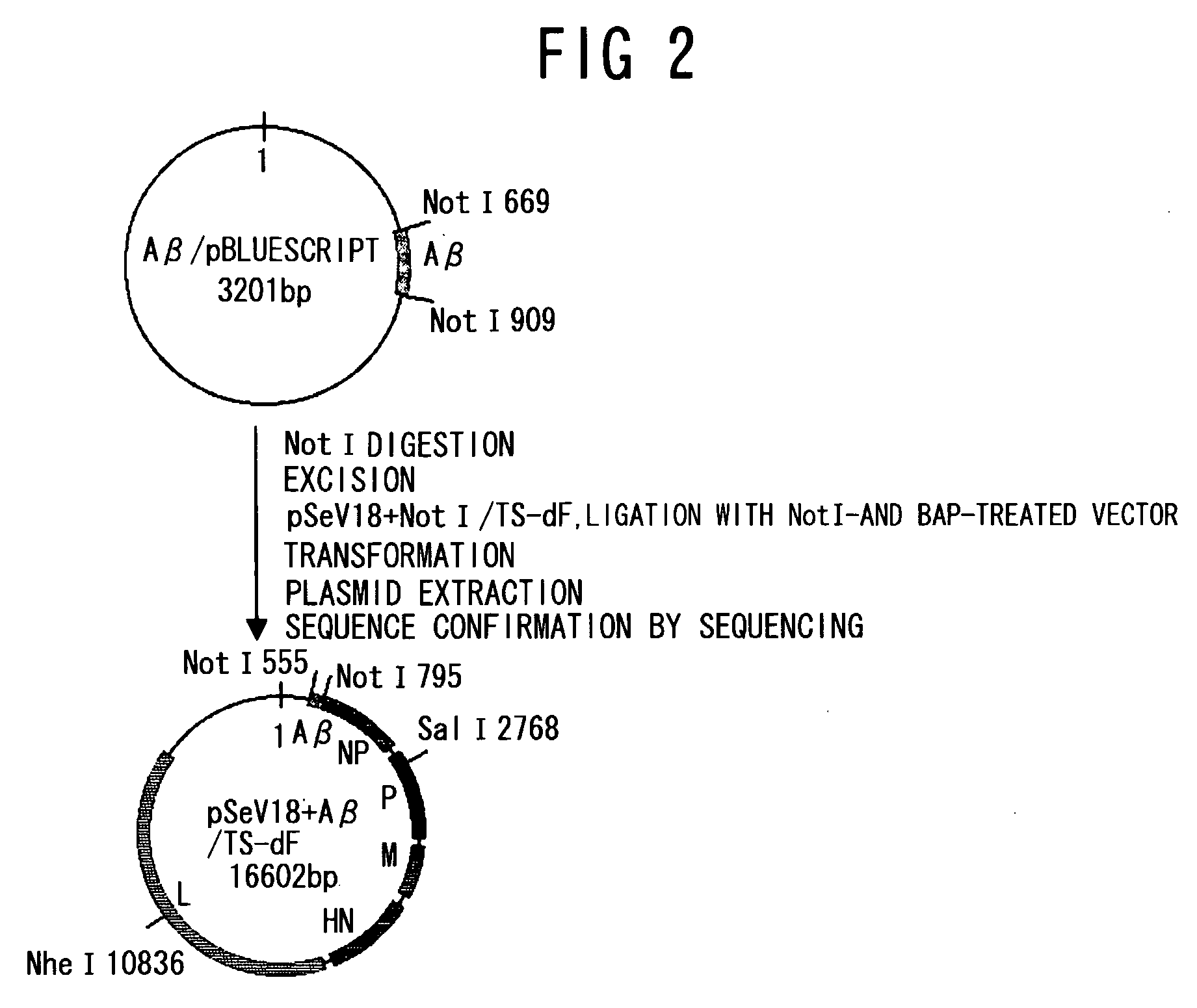
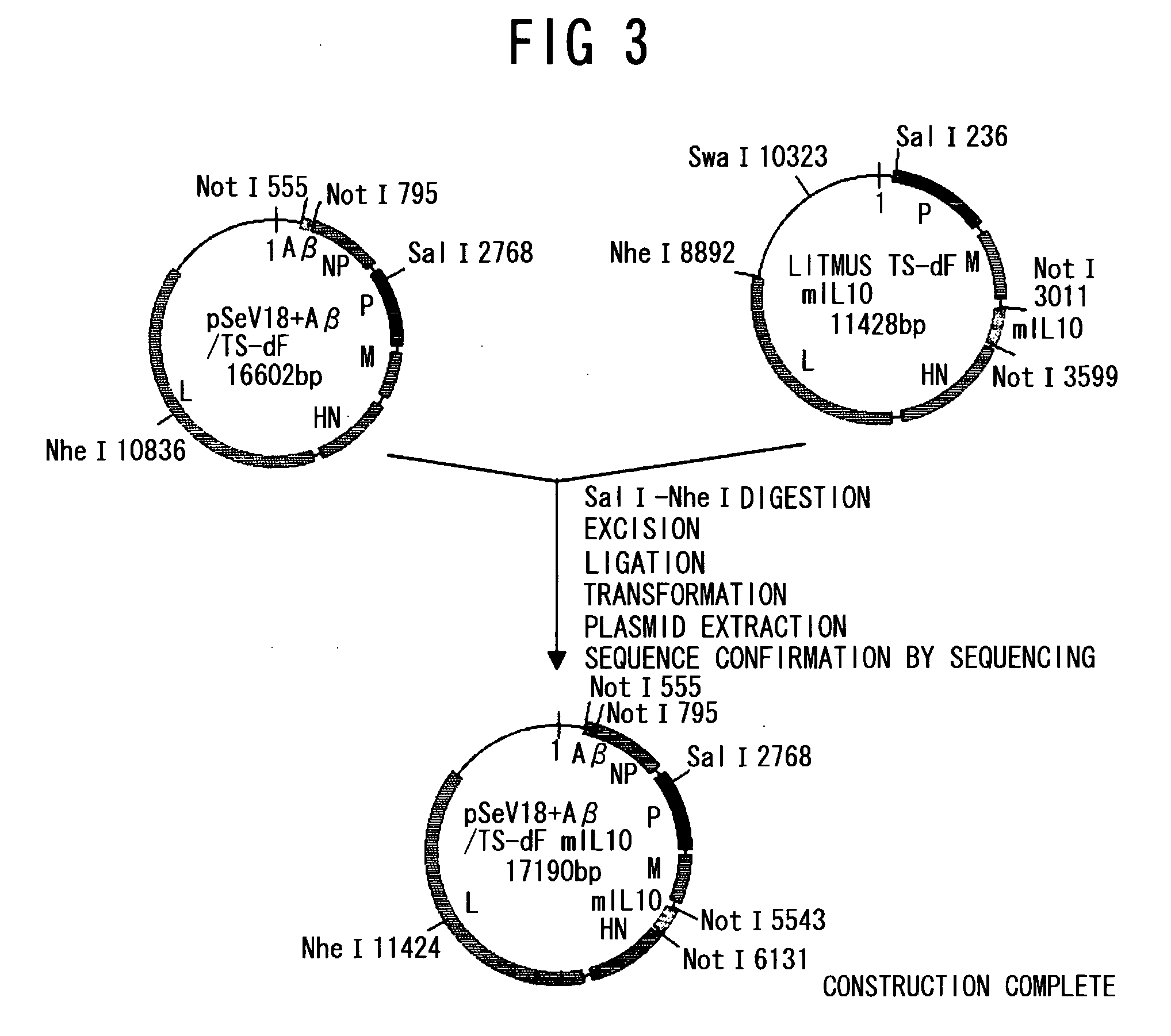
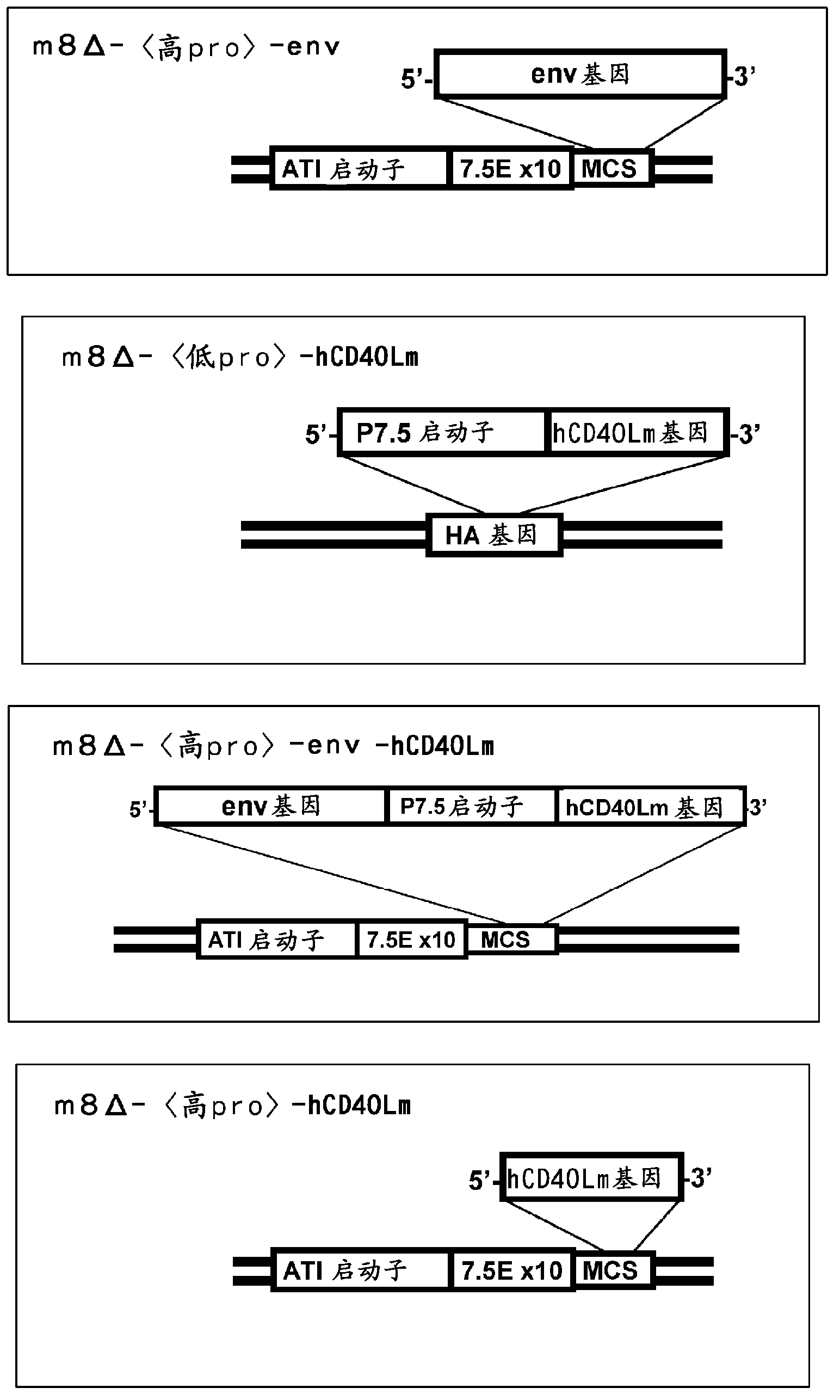
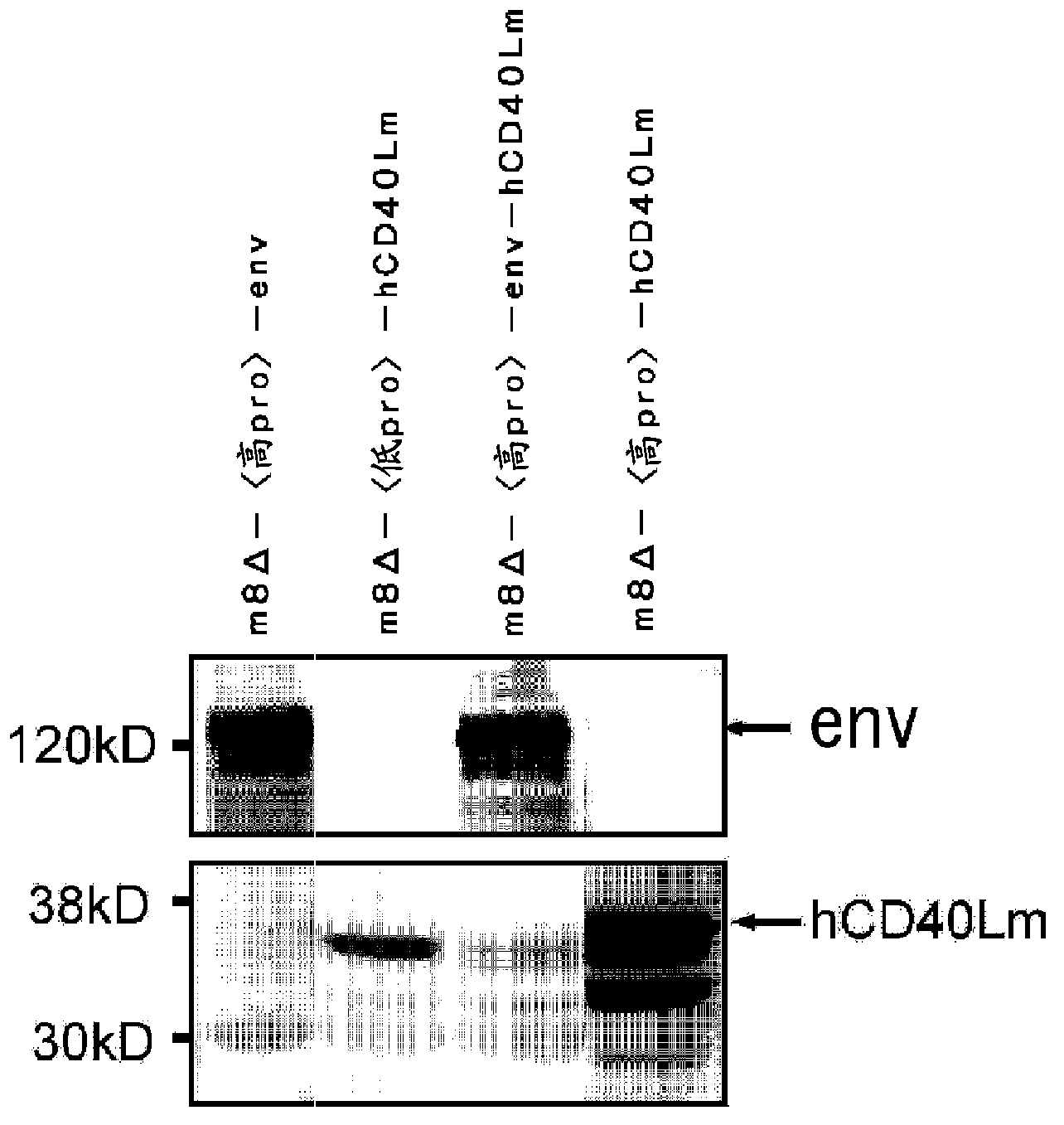
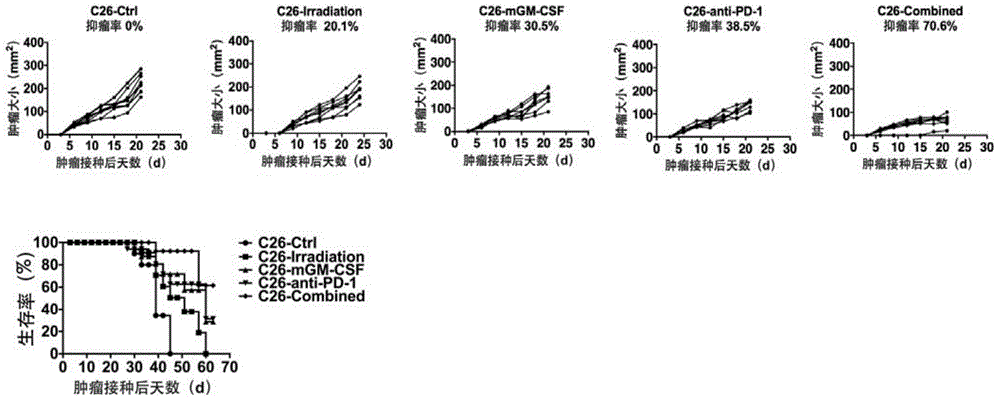
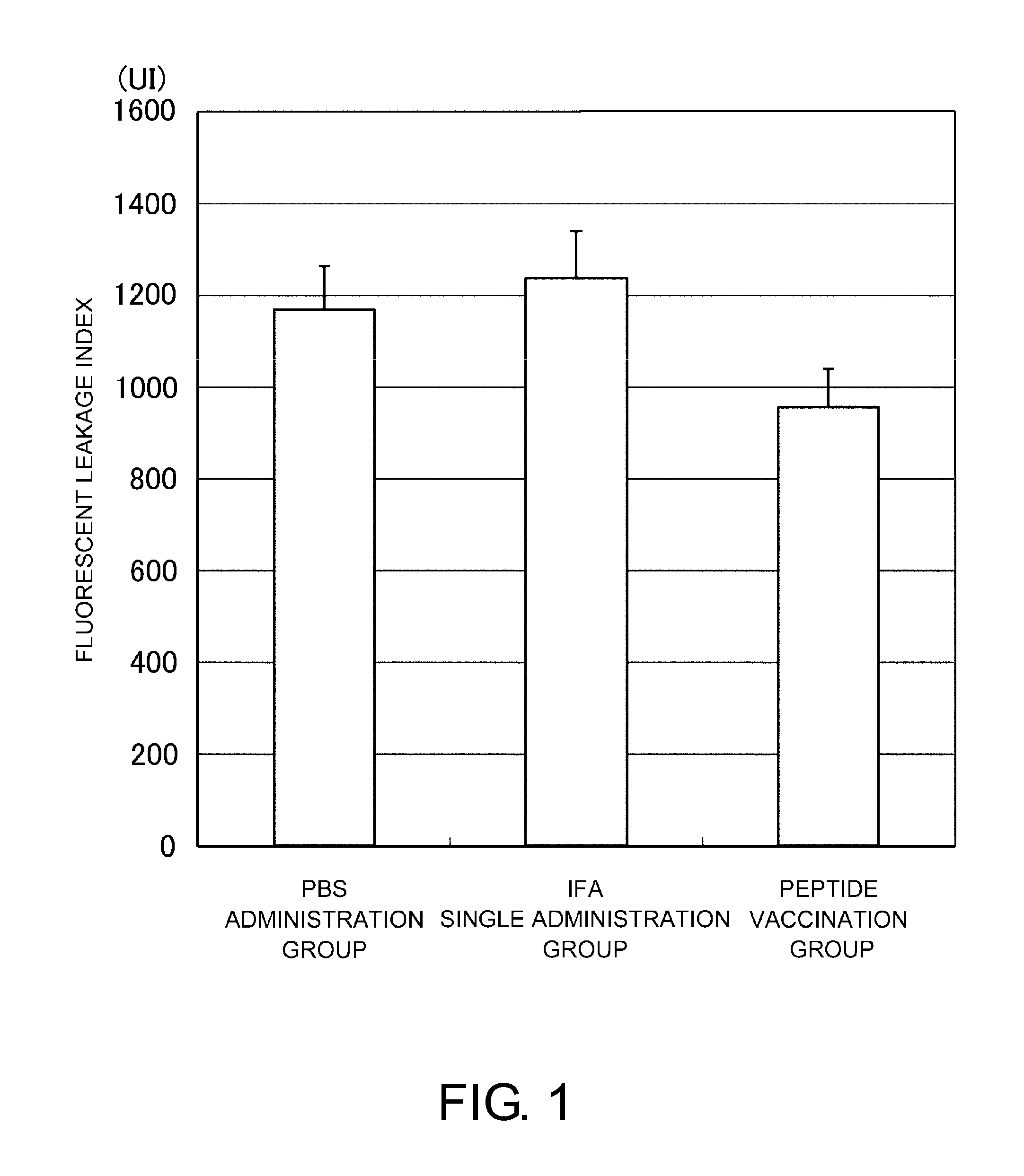
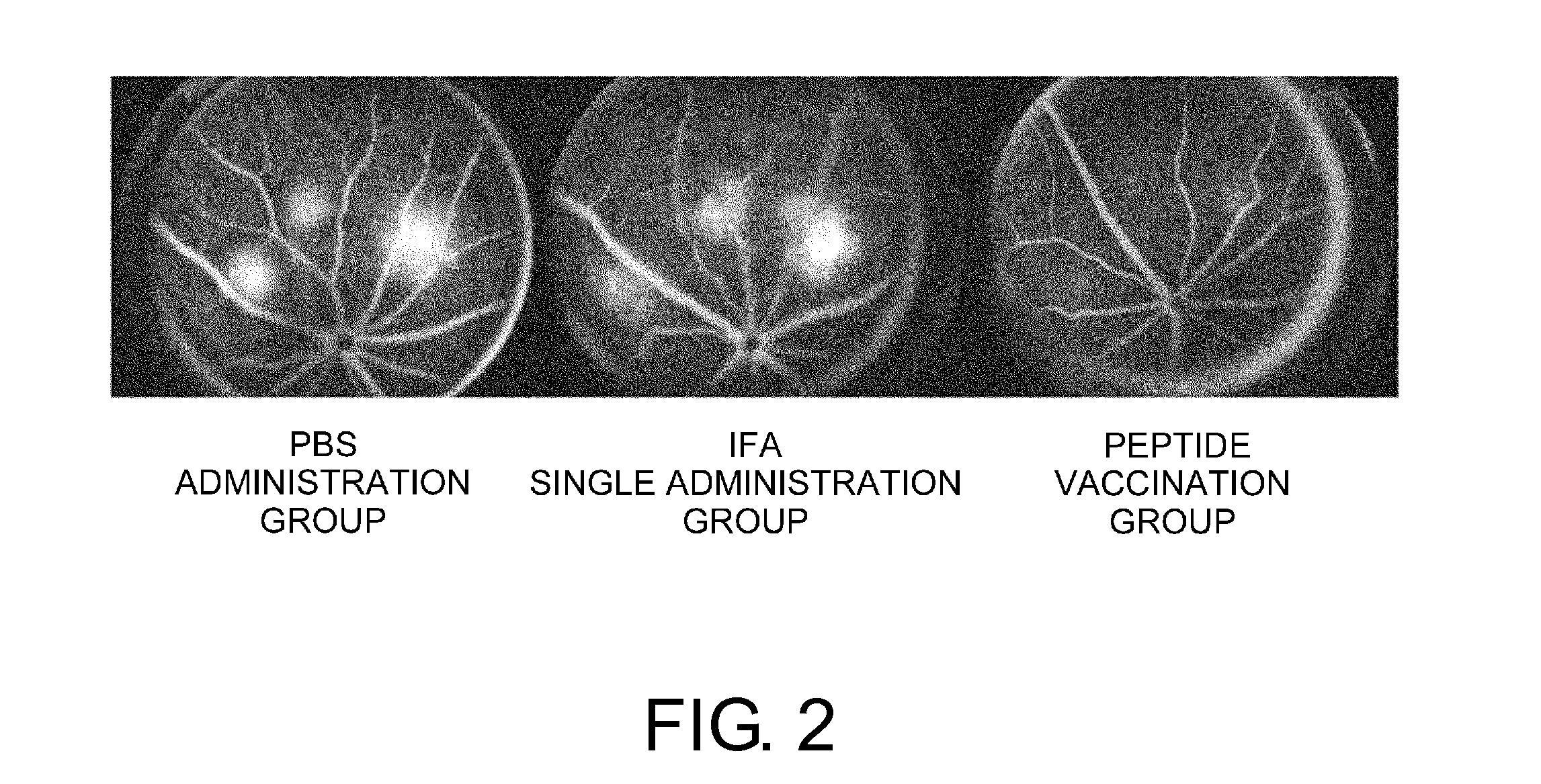
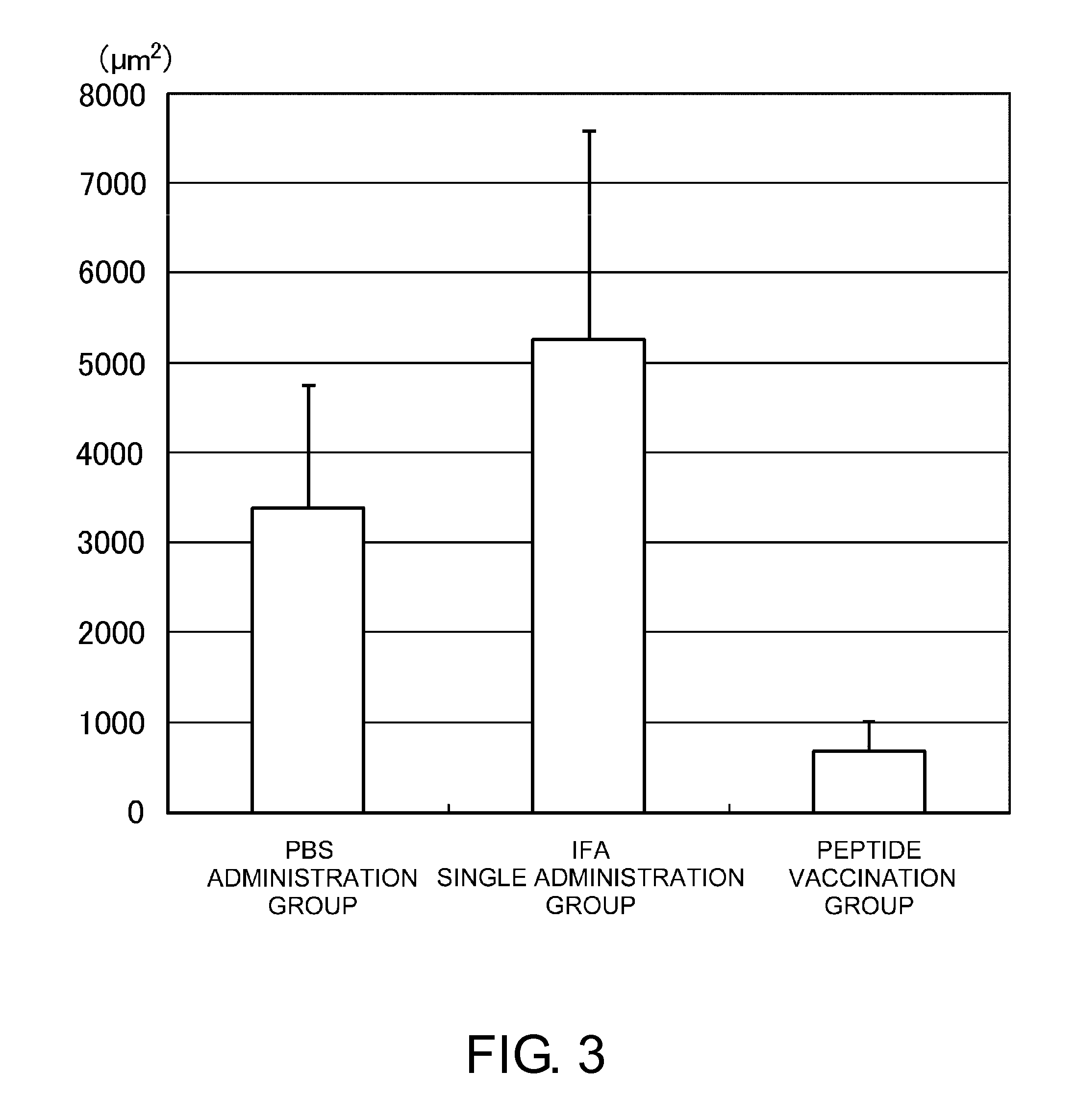
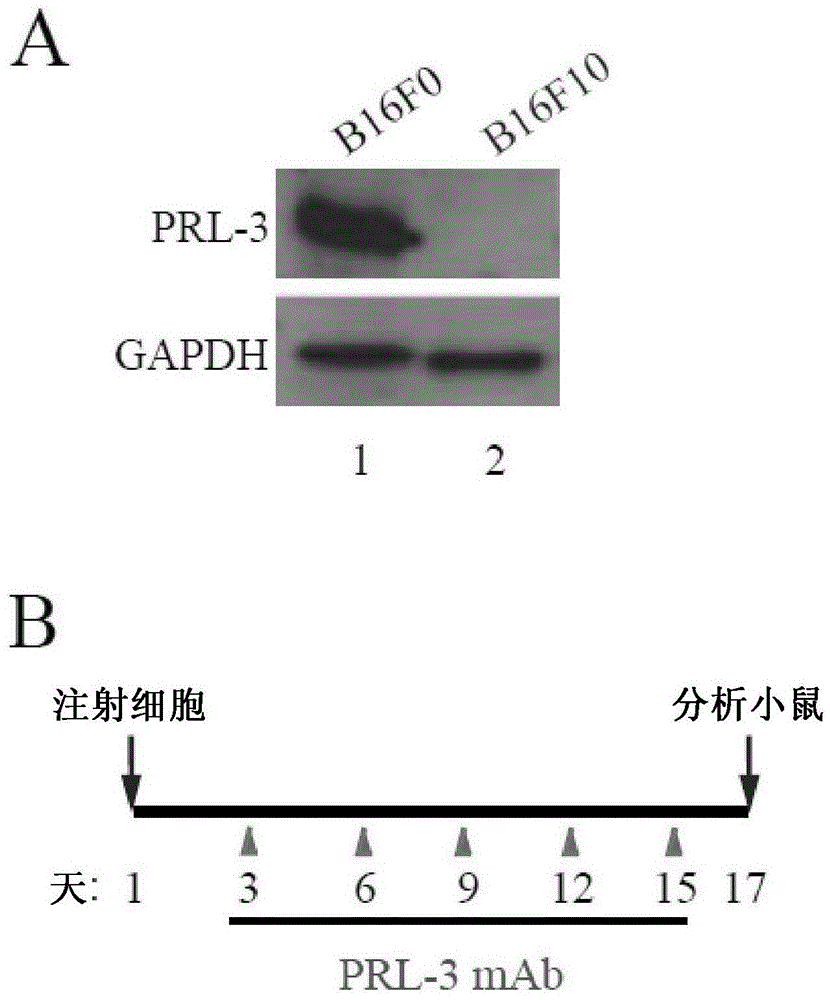
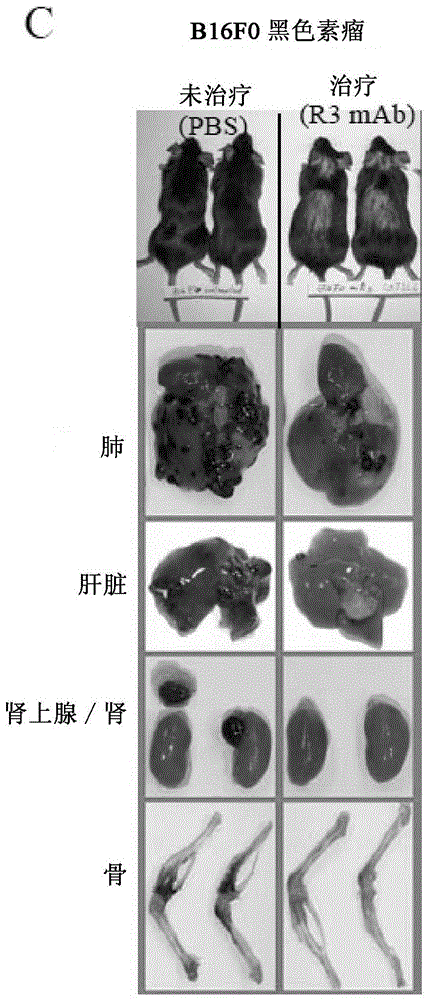
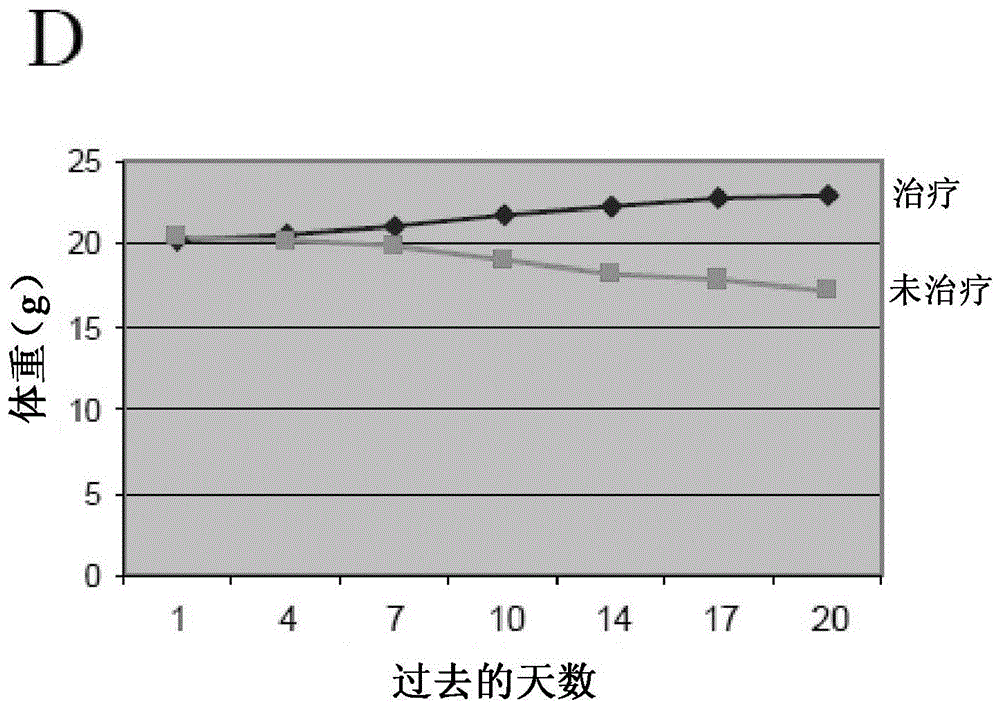
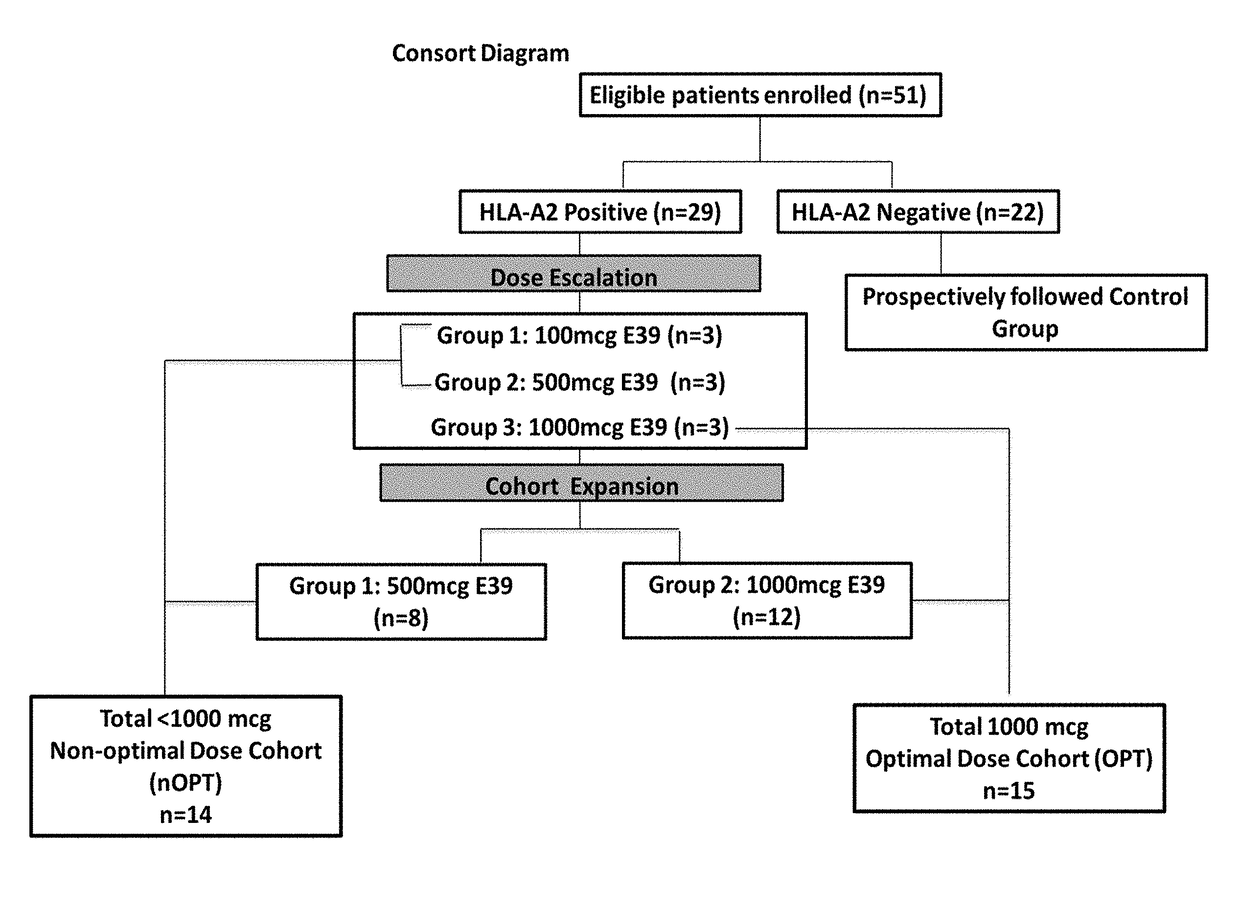
Patents
Literature
33 results about "Vaccine therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vaccine therapy is a type of treatment that uses a substance or group of substances to stimulate the immune system to destroy a tumor or infectious microorganisms such as bacteria or viruses.
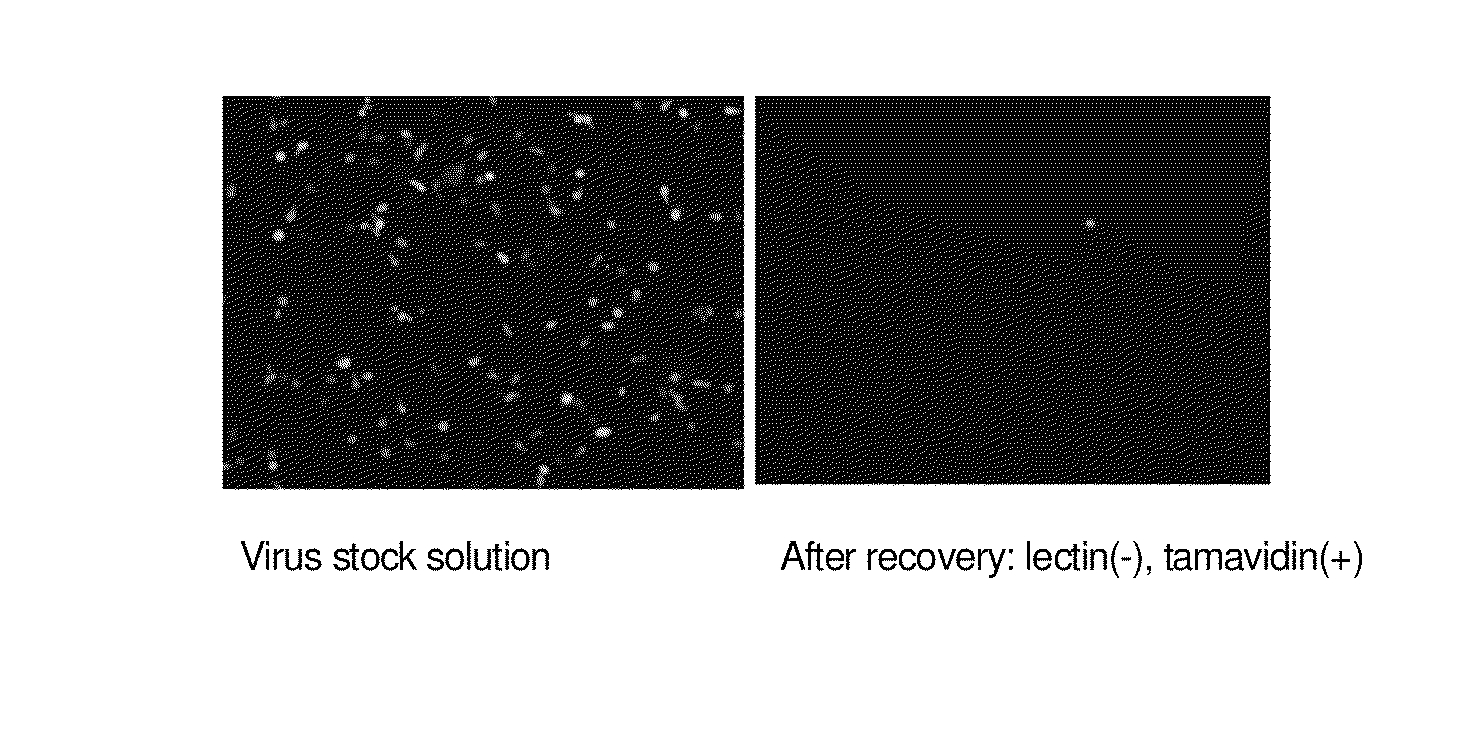
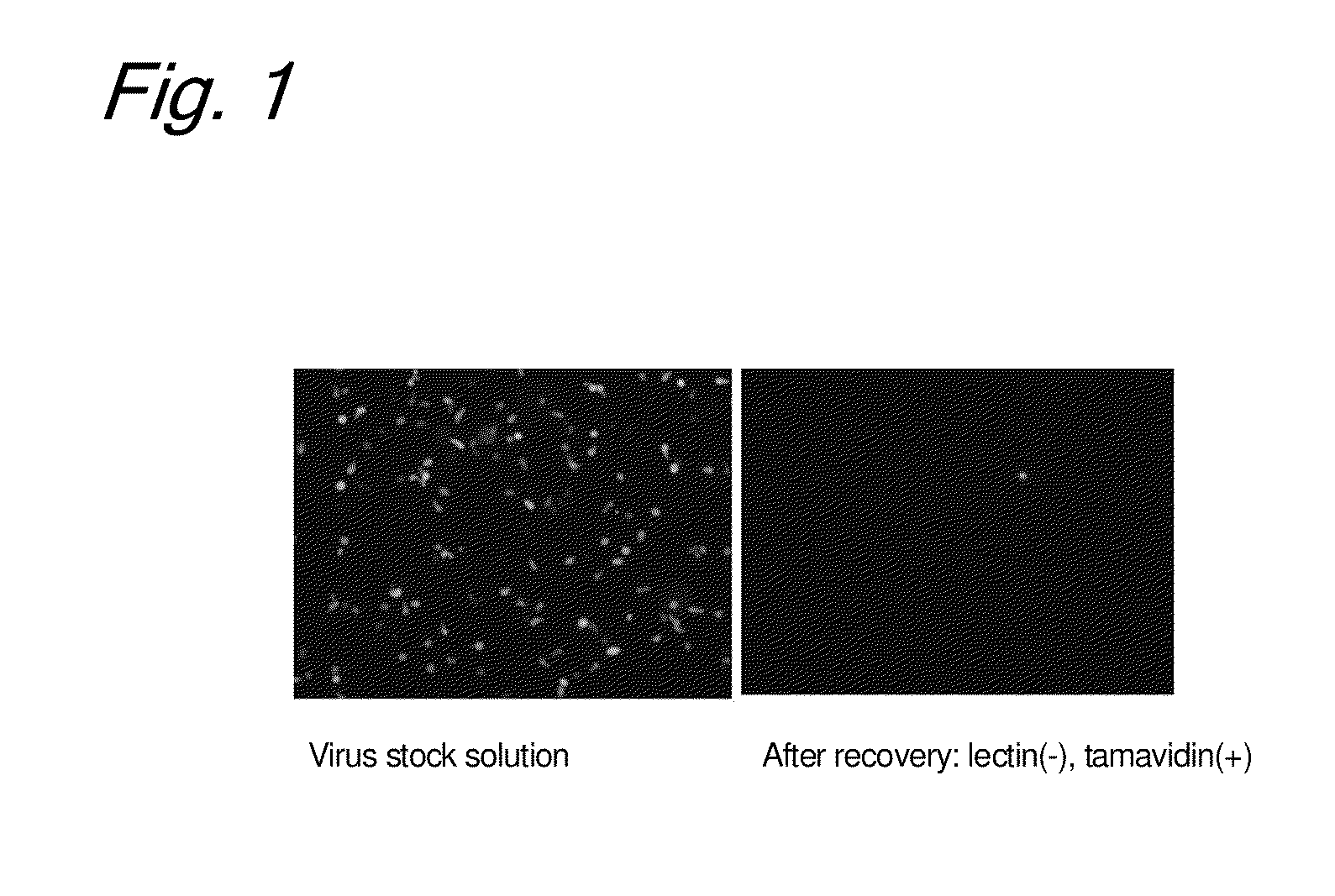
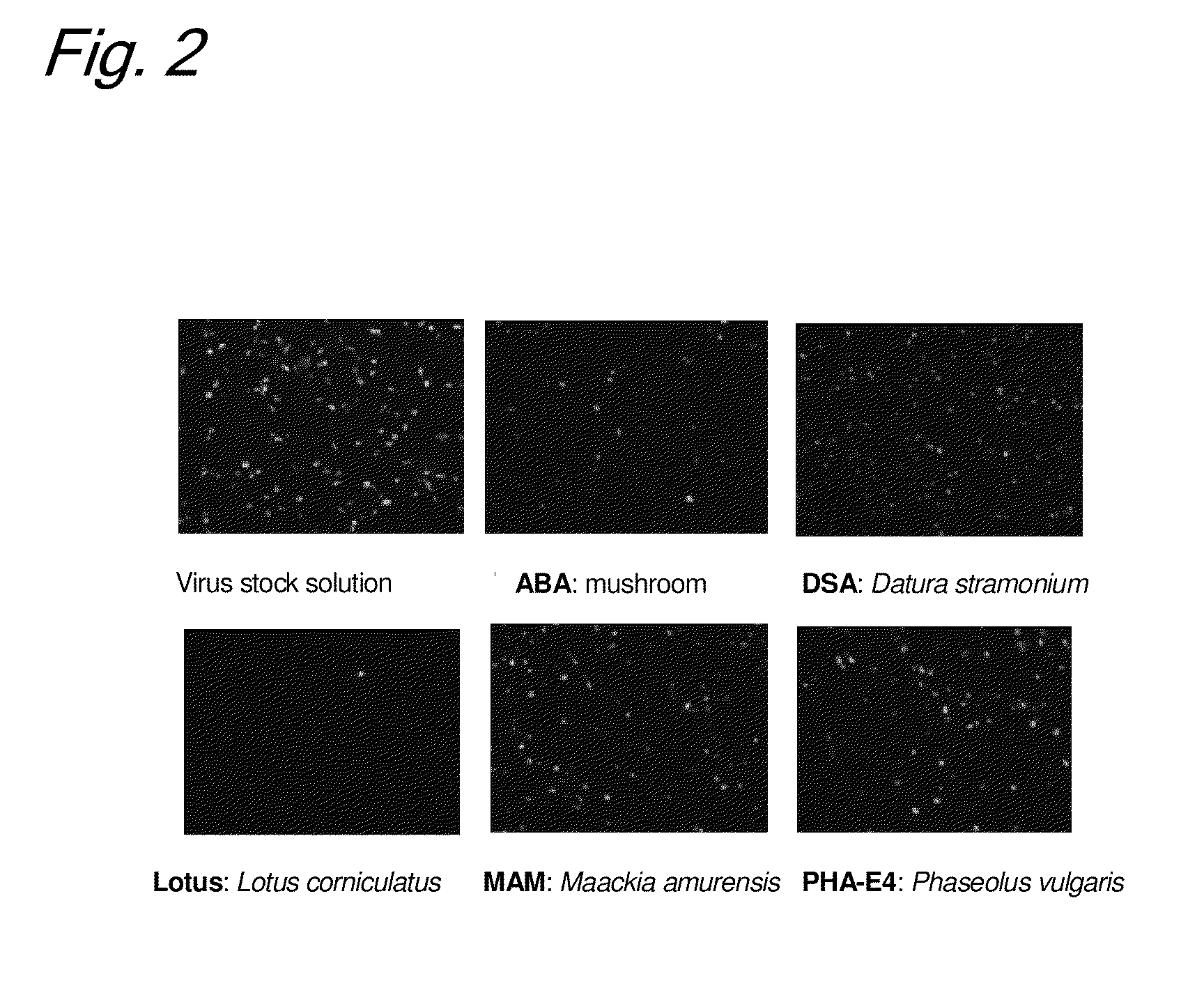
Enrichment method of virus
InactiveUS20110053250A1Maintaining their infection abilityImprove concentrationBiological material analysisRecovery/purificationEnrichment methodsViral vector
The present invention provides a novel method that can increase readily a virus or viral vector concentration in a solution having a low concentration and a kit for performing the method. Conventional methods require complicated operations, expensive equipment, or highly trained experts for efficiently concentrating viruses from low-concentration virus solutions. The method of the present invention can concentrate viral vectors readily while maintaining infection abilities of the viral vectors, and thus it can be used as a safe and simple technique for concentrating a vector useful in the field of a genetic therapy or a vaccine therapy using a viral vector.
Owner:JAPAN TOBACCO INC +1
Highly safe intranasally administrable gene vaccines for treating alzheimer's disease
InactiveUS20090170798A1Large scaleLow costSsRNA viruses negative-senseOrganic active ingredientsDiseaseNose
An objective of the present invention is to provide a safe and effective vaccine therapy for Alzheimer's disease. A minus strand RNA viral vector carrying amyloid gene was constructed, and administered intranasally to 24- to 25-months-old APP transgenic mice. The level of serum anti-A 42 antibody was determined and showed to be markedly higher than the control. The results of histological investigation showed that the administration of a vector of the present invention markedly reduced senile plaques in all of the frontal lobe, parietal lobe, and hippocampus. The brain A level was also markedly reduced. Furthermore, the administration of a vector of the present invention did not result in lymphocyte infiltration in the central nervous system.
Owner:DNAVEC CORP +1
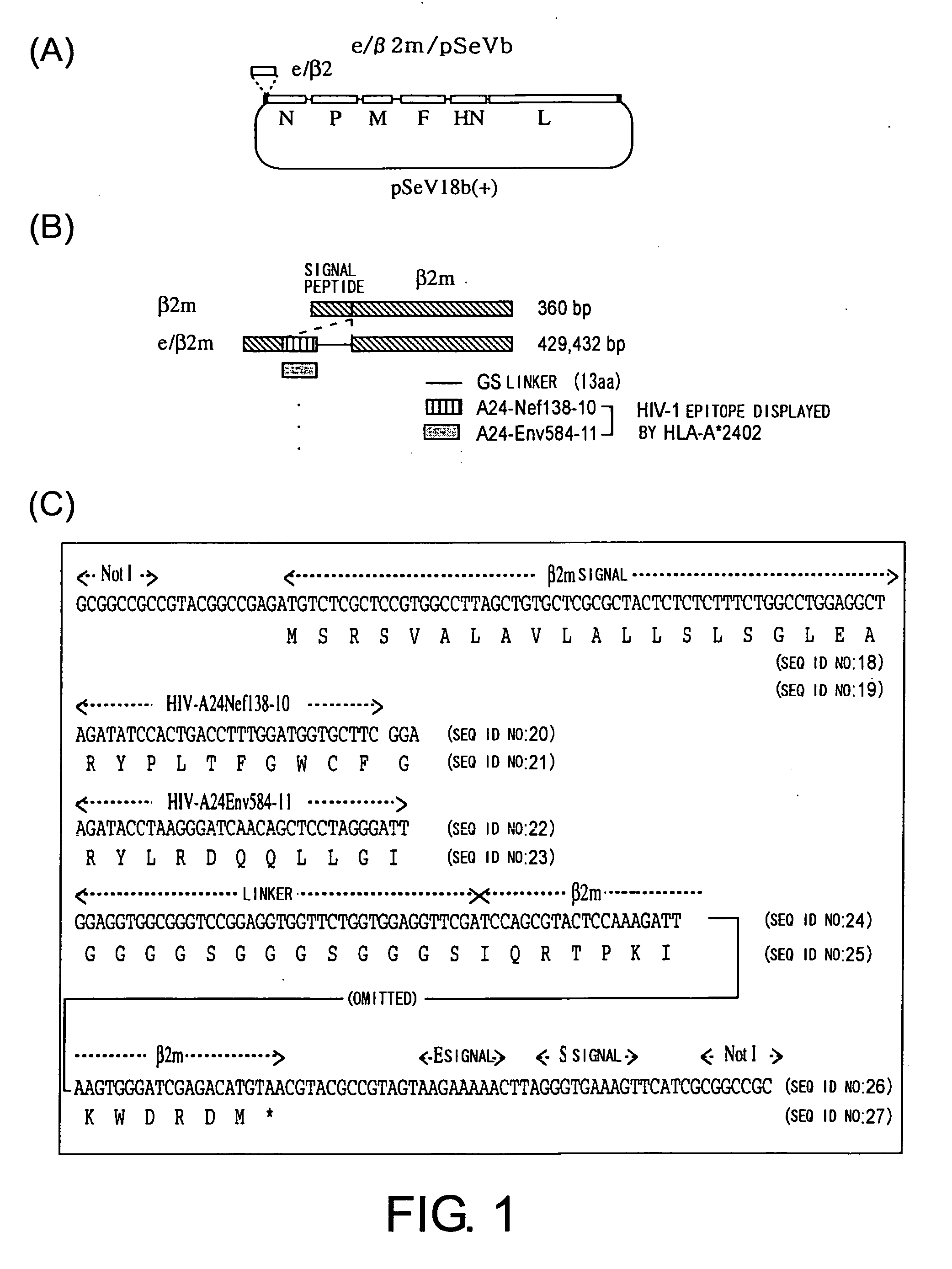
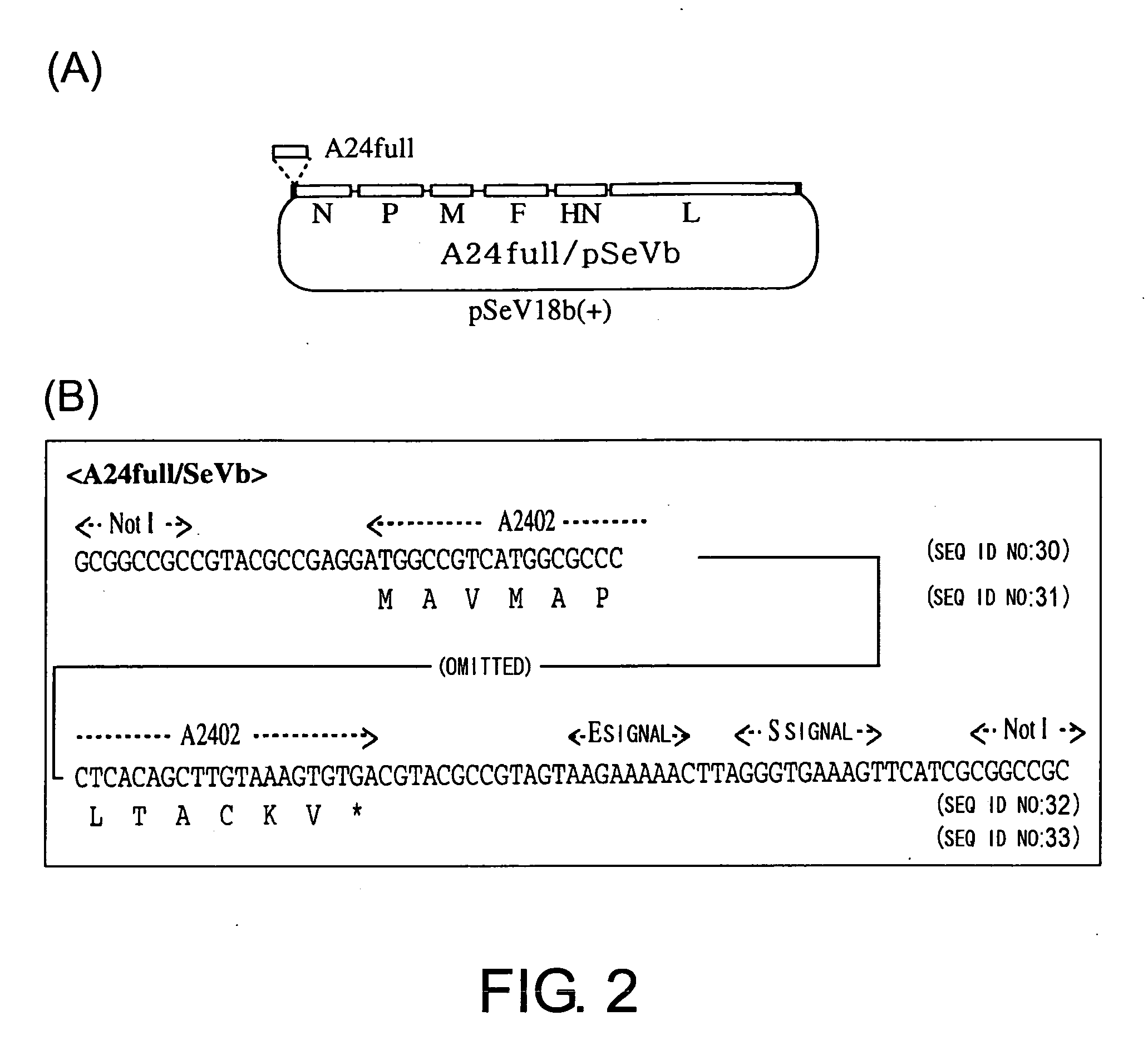
Virus vector for prime/boost vaccines, which comprises vaccinia virus vector and sendai virus vector
InactiveCN103189506APrevention is effectiveEffective treatmentSsRNA viruses negative-senseViral antigen ingredientsPathogenic microorganismPox Virus Vector
To provide a virus vector which can be used for producing a prime / boost vaccine that can activate both cellular immunity and humoral immunity and is effective on infections by pathogenic microorganisms and malignant tumors which are generally believed to be difficult to be treated by vaccine therapy. Provided is a virus vector for prime / boost vaccines, comprising the following components (a) and (b): (a) a vaccinia virus vector which carries a gene encoding an immunogenic polypeptide in such a manner that the gene can be expressed; and (b) a Sendal virus vector which carries the gene encoding the immunogenic polypeptide in such a manner that the gene can be expressed.
Owner:HOKKAIDO UNIVERSITY
Production of Homogeneous Cell Line Highly Permissive To Porcine Circovirus Type 2 (PCV2) Infection
Continuous cell lines that are highly permissive to infection by porcine circovirus type 2 (“PCV2”) are described. PCV2 is the causal agent of post-weaning multi-systemic wasting syndrome (“PMWS”) in pigs. PMWS has emerged as a major disease that poses a significant threat to the economics of global swine industry. The highly permissive cell lines of this invention provide efficient and reliable sources of PCV2 for use in development of vaccines, therapies and diagnostic agents for PMWS.
Owner:TEMASEK LIFE SCIENCES LABORATORY
Virus vector for prime/boost vaccines, which comprises vaccinia virus vector and sendai virus vector
InactiveUS20130302367A1Avoid infectionEffective preventionSsRNA viruses negative-senseViral antigen ingredientsCowpox virusPrime boost
[Problem]To provide a set of virus vectors which can be used for producing a prime / boost vaccine that can activate both cellular immunity and humoral immunity and is effective on infections by pathogenic microorganisms and malignant tumors which are generally believed to be difficult to be treated by vaccine therapy.[Solution]Provided is a set of virus vectors for prime / boost vaccines, comprising the following virus vector (a) and virus vector (b): (a) a vaccinia virus vector which carries a gene encoding an immunogenic polypeptide in such a manner that the gene can be expressed; and (b) a Sendal virus vector which carries the gene encoding the immunogenic polypeptide in such a manner that the gene can be expressed.
Owner:HOKKAIDO UNIVERSITY +1
Tumor cell vaccine simultaneously secreting PD-1 neutralizing antibody and GM-CSF factor and preparation method thereof
InactiveCN105031630AGood anti-tumor effectLifting of immunosuppressionAntibody ingredientsImmunological disordersTreatment fieldTherapeutic effect
The invention discloses the field of tumor cellular immunotherapy and particularly relates to a tumor cell vaccine which is modified by a PD-1 neutralizing antibody with combination of a GM-CSF factor and a preparation method thereof. In the invention, tumor cellular vaccine therapy is creatively combined with antibody therapy which relieves tumor immune-suppression, so that the prepared tumor cell vaccine simultaneously secreting the PD-1 neutralizing antibody and the GM-CSF factor can not only relieve the immune-suppression status of a tumor micro-environment but also enhance an antitumor immune reaction. The tumor cell vaccine is good in antitumor treatment effect, is excellent in development value and provides a new approach of tumor cellular immunotherapy.
Owner:SICHUAN UNIV
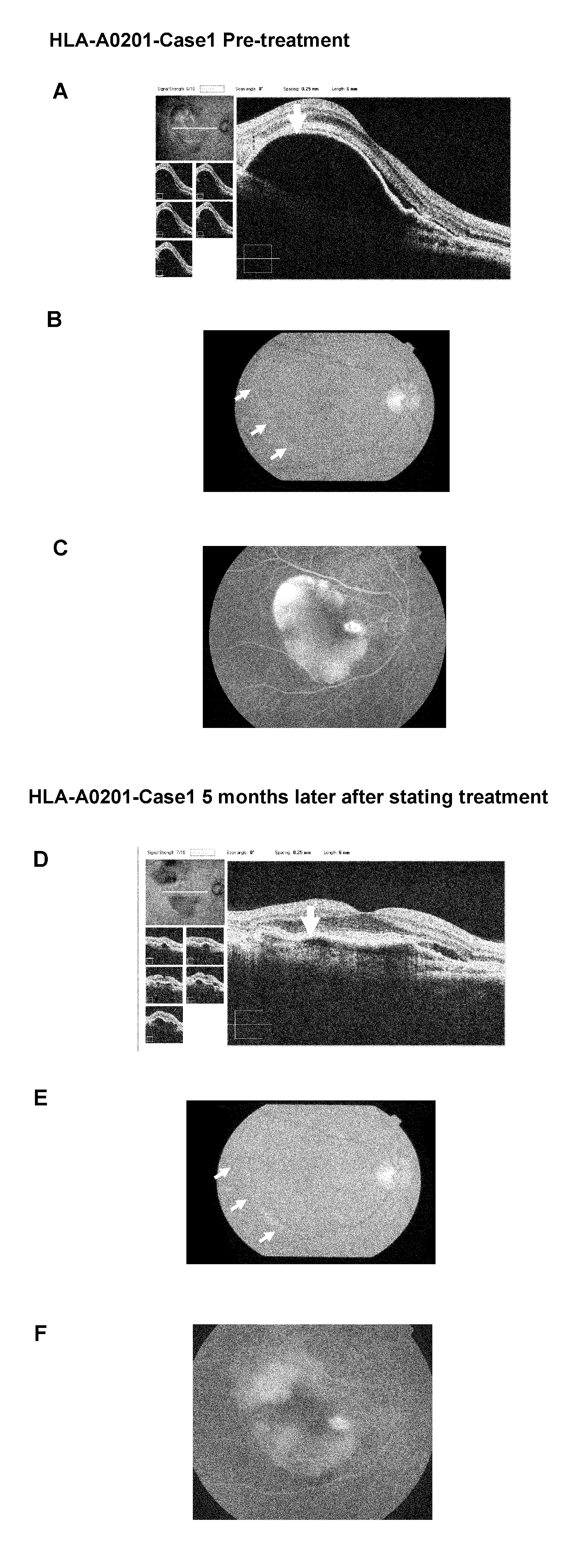
Vaccine therapy for choroidal neovascularization
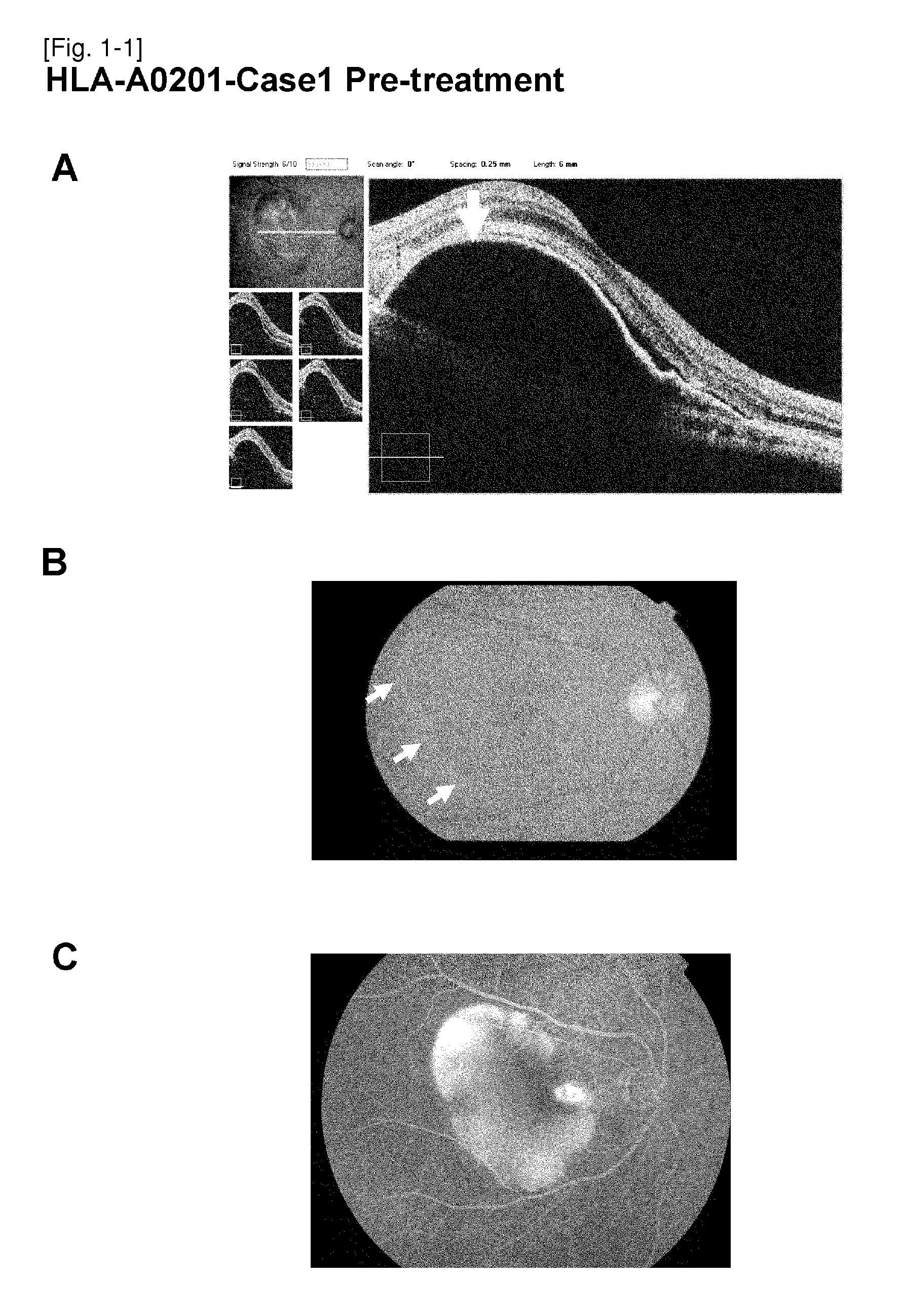
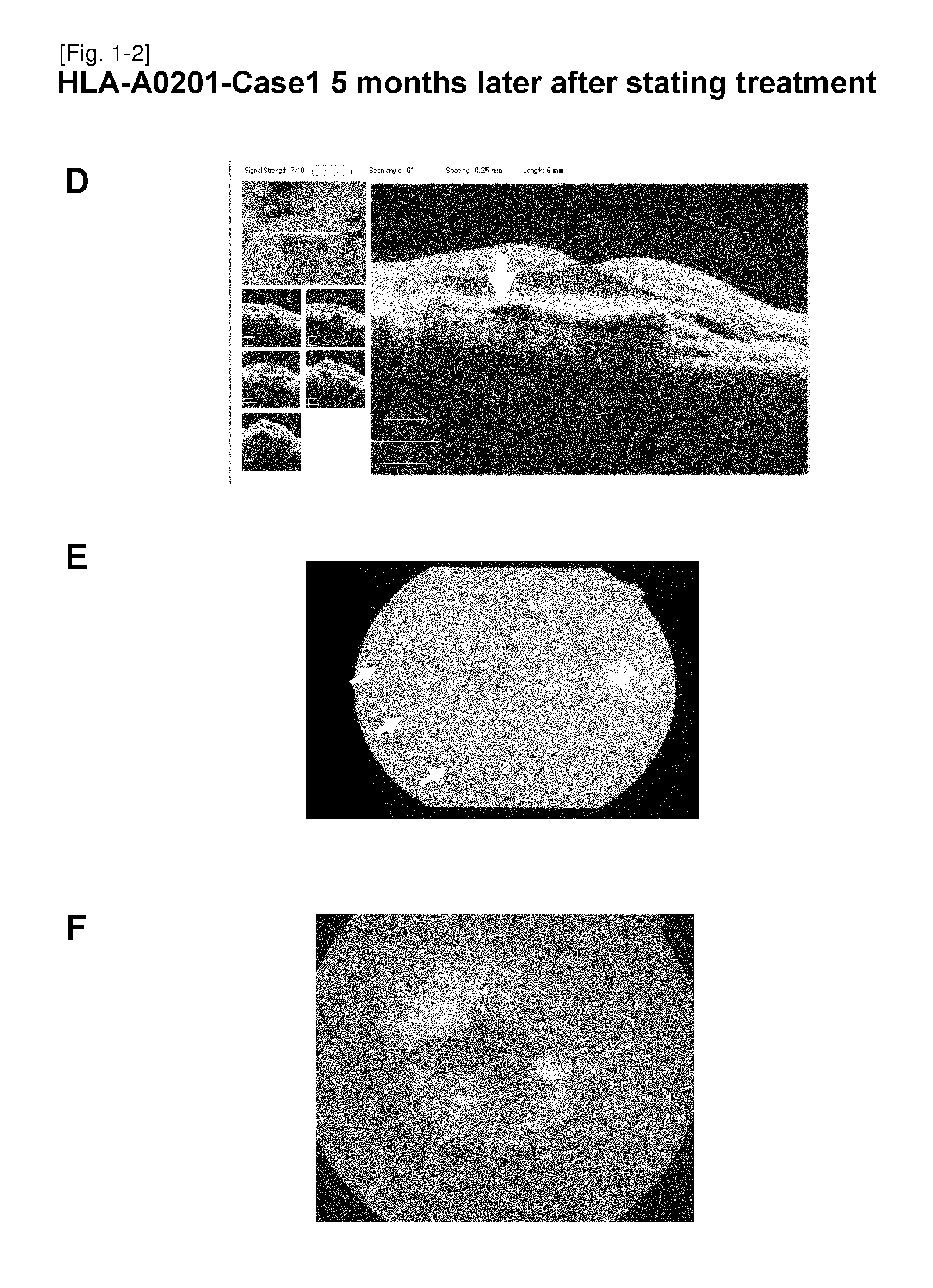
The present inventors administered a peptide derived from VEGFR-2, which is known as one of the proteins involved in neovascularization, to model mice (A2 / Kb transgenic mice) expressing human HLA-A*0201, and tested whether or not the peptide has vaccine effect. As a result, the present inventors successfully discovered that vaccination using this peptide as an antigen is effective for inhibition of choroid neovascularization, and thereby completed the present invention. More specifically, the present invention provides vaccines for treatment and / or prevention of diseases caused by choroid neovascularization (neovascular maculopathy), which contain a VEGFR-2-derived peptide as an active ingredient.
Owner:ONCOTHERAPY SCI INC
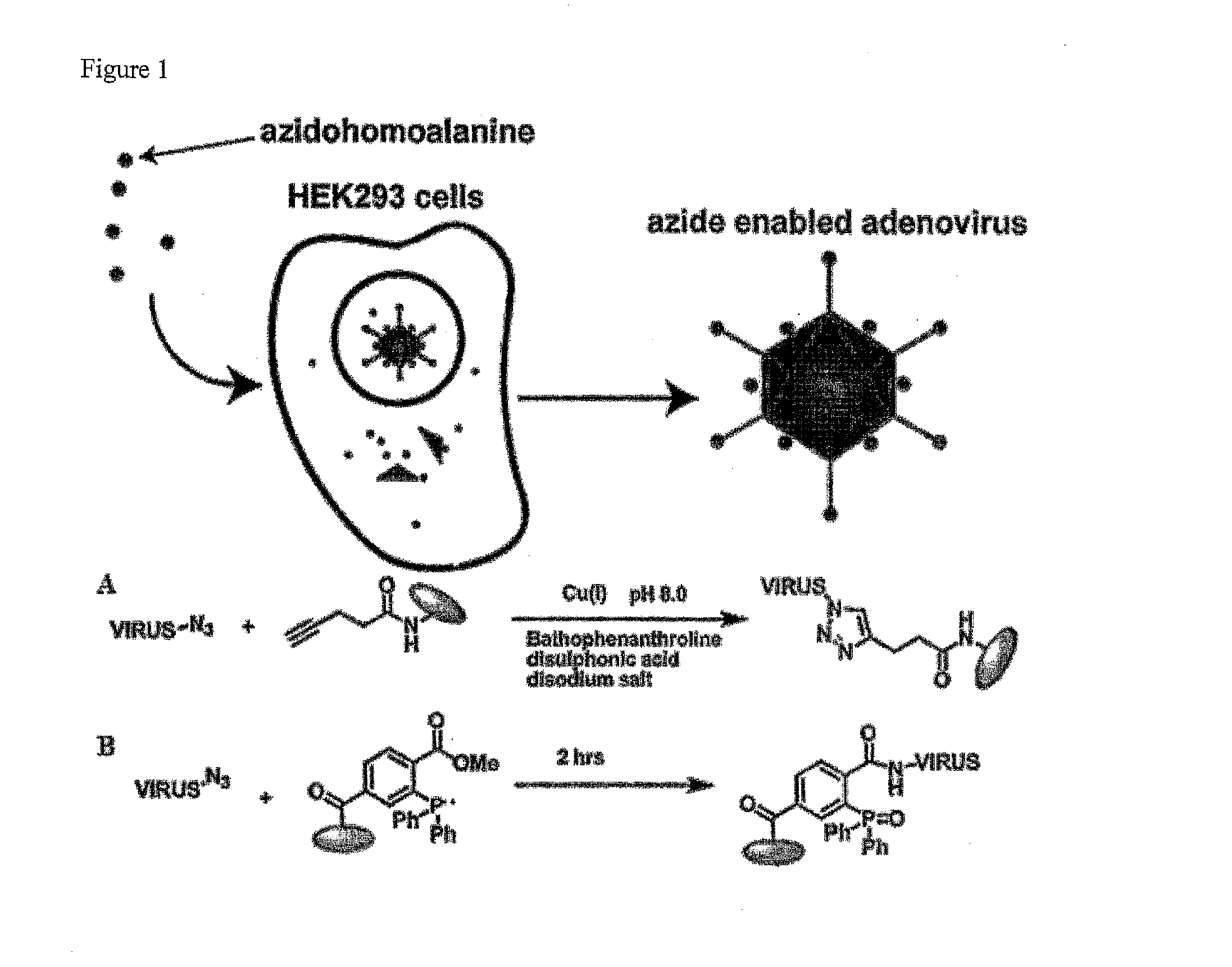
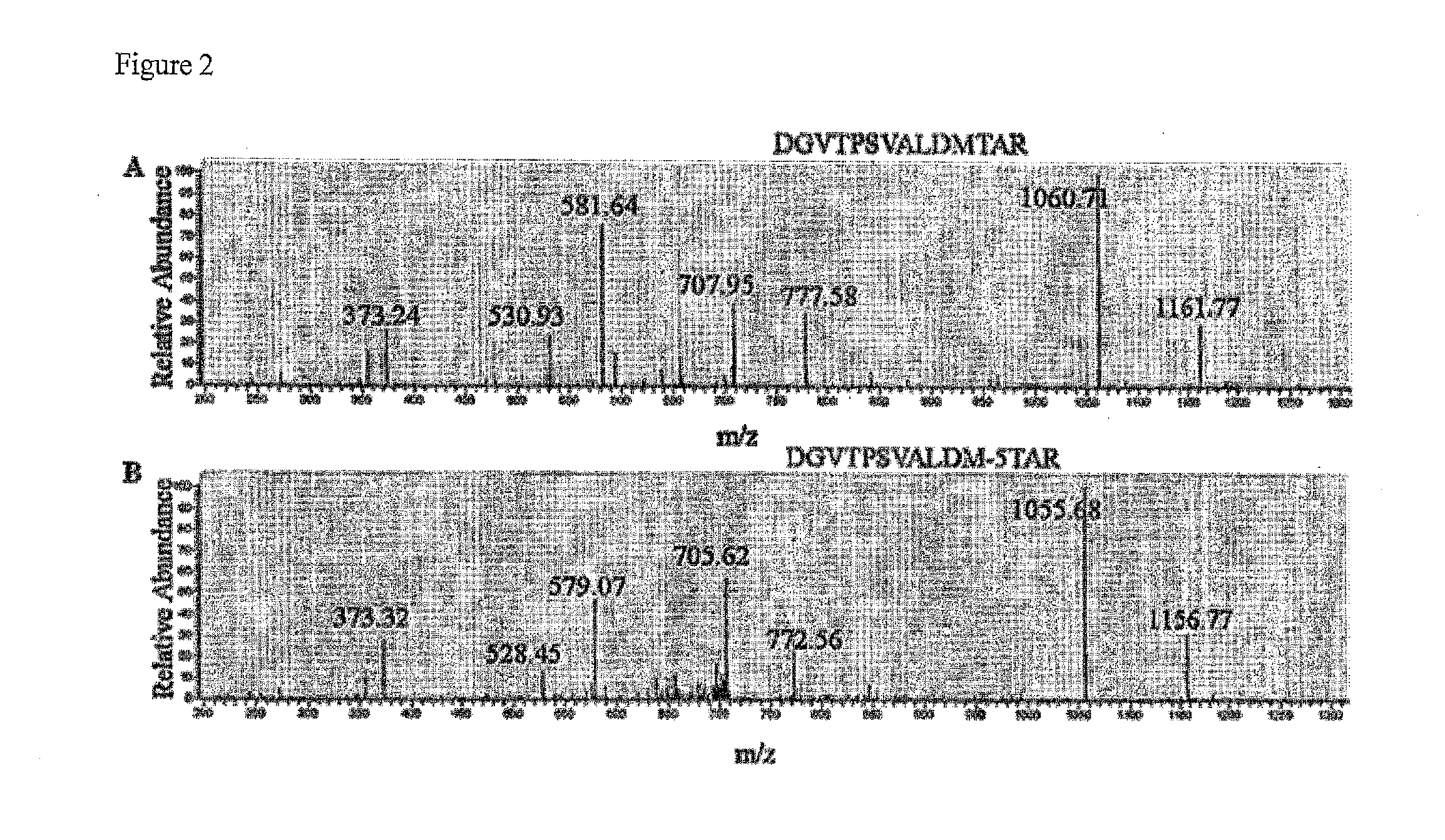
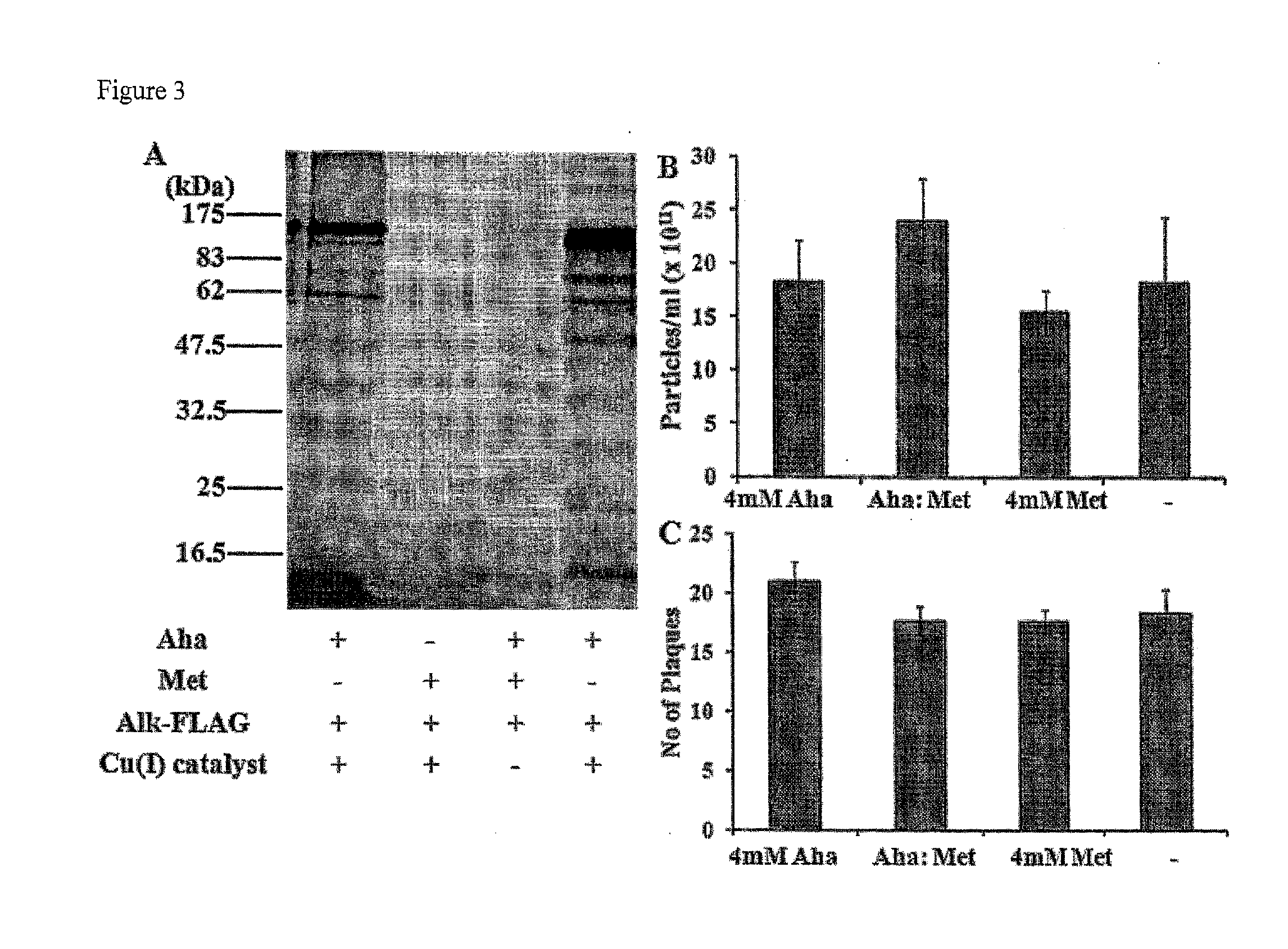
Viruses modified with unnatural moieties and methods of use thereof
The invention provides compositions and methods for making and using modified viruses, including infectious viruses, having an external surface linked to at least one heterologous unnatural moiety that is exemplified by unnatural amino acid and unnatural saccharide. The unnatural moiety that is linked to the invention's modified viruses is optionally further linked to a molecule of interest (such as probe, cytotoxin, therapeutic molecule, antibody, affibody, epitope, etc. The invention's compositions and methods for use in, for example, diagnostic applications and therapeutic applications such as gene therapy, oncolytic therapy, and / or vaccine therapy.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Vaccine therapy for choroidal neovascularization
The present invention provides novel pharmaceutical agents and methods for treating or preventing diseases caused by neovascularization in human choroid (neovascular maculopathy). The present invention provides pharmaceutical compositions and vaccines for treating and / or preventing diseases caused by neovascularization in human choroid (neovascular maculopathy), comprising at least one type each of a peptide comprising an amino acid sequence derived from a VEGFR-1 protein and having an activity of inducing cytotoxic T cells, and a peptide comprising an amino acid sequence derived from a VEGFR-2 protein and having an activity of inducing cytotoxic T cells.
Owner:ONCOTHERAPY SCI INC
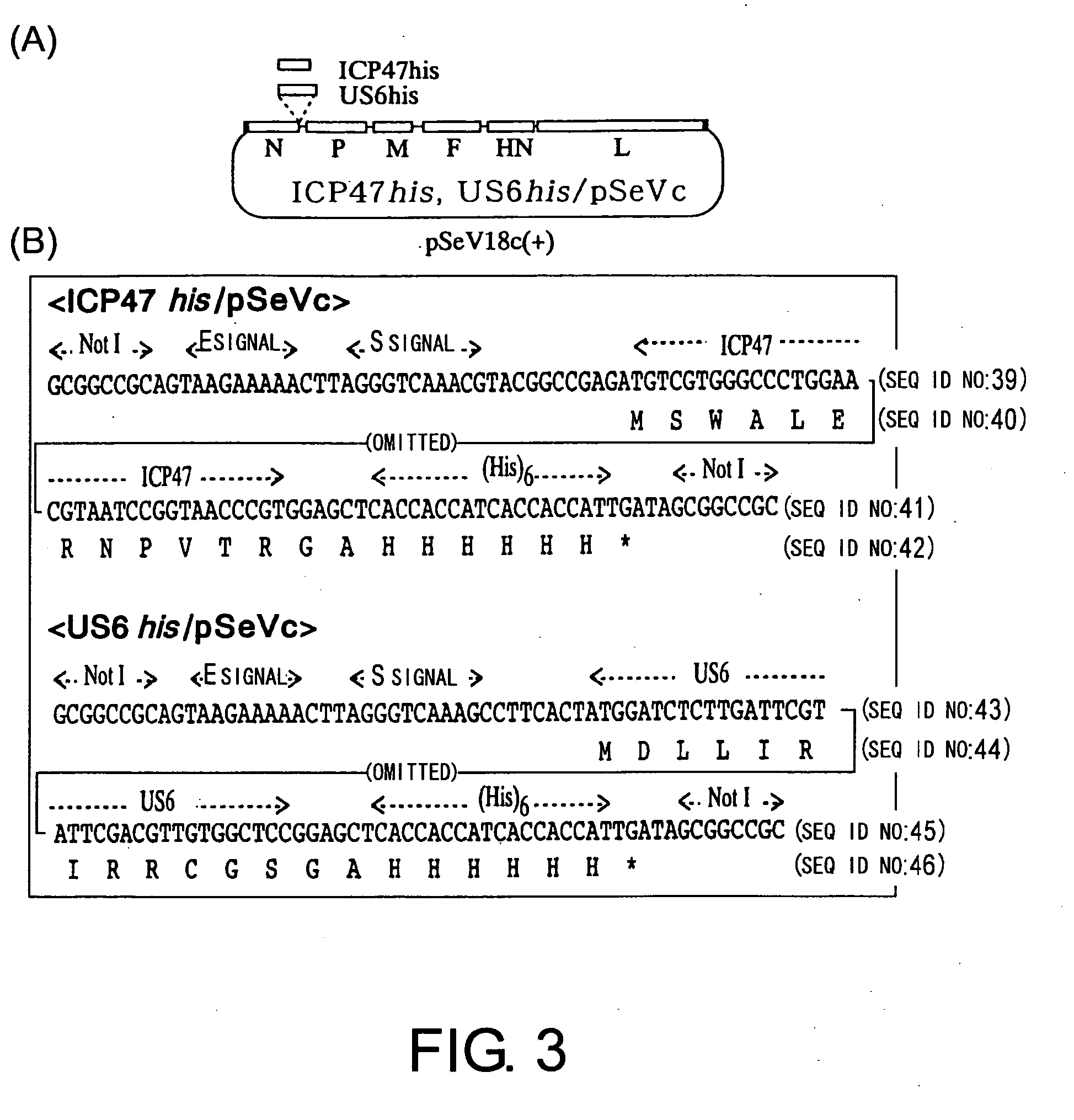
Method of strengthening foreign epitope presentation by mhc class i by inhibiting tap activity
InactiveUS20060099218A1Easy accessHigh frequencyAntibody mimetics/scaffoldsVirus peptidesMHC class IViral vector
Owner:DNAVEC RES
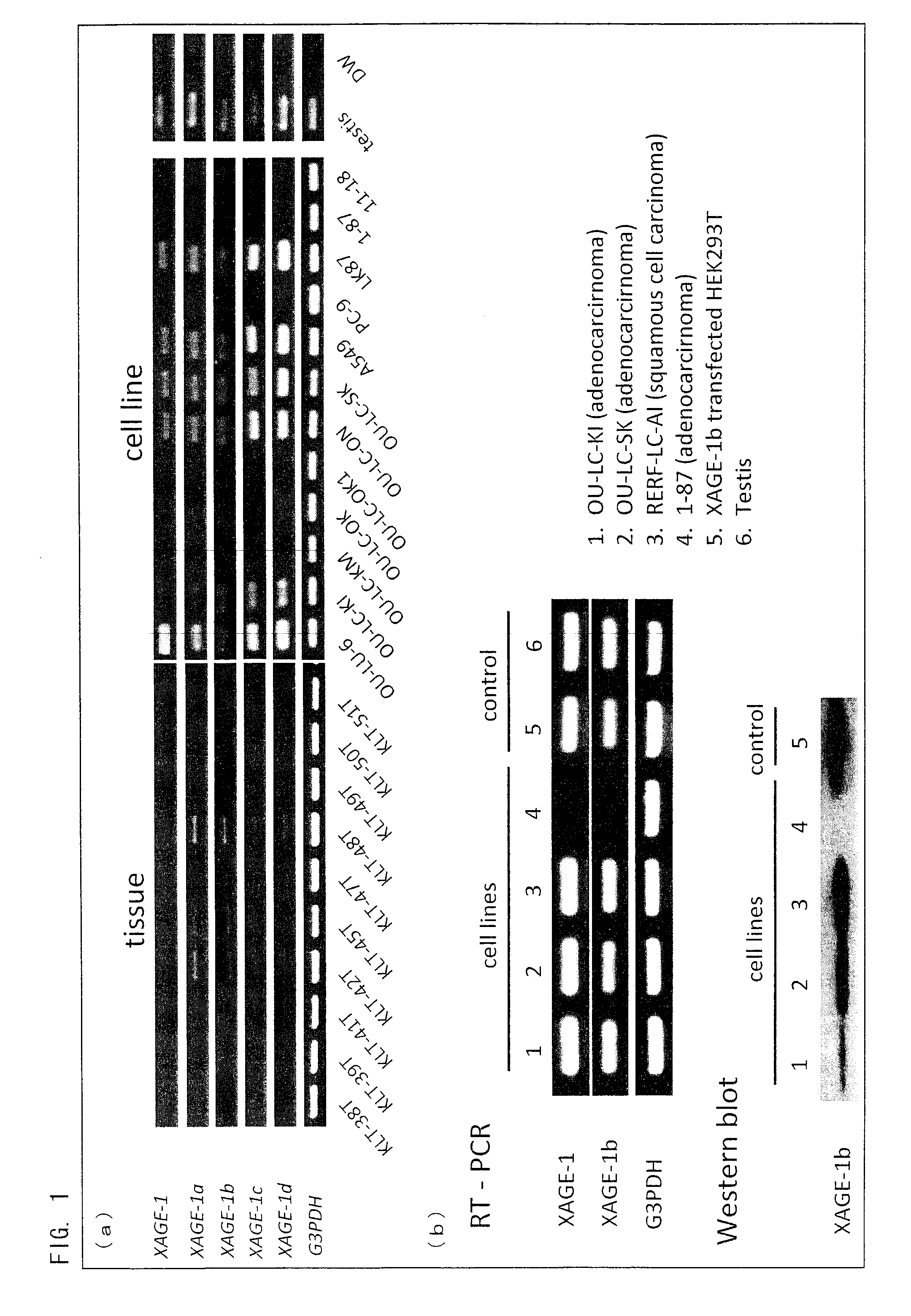
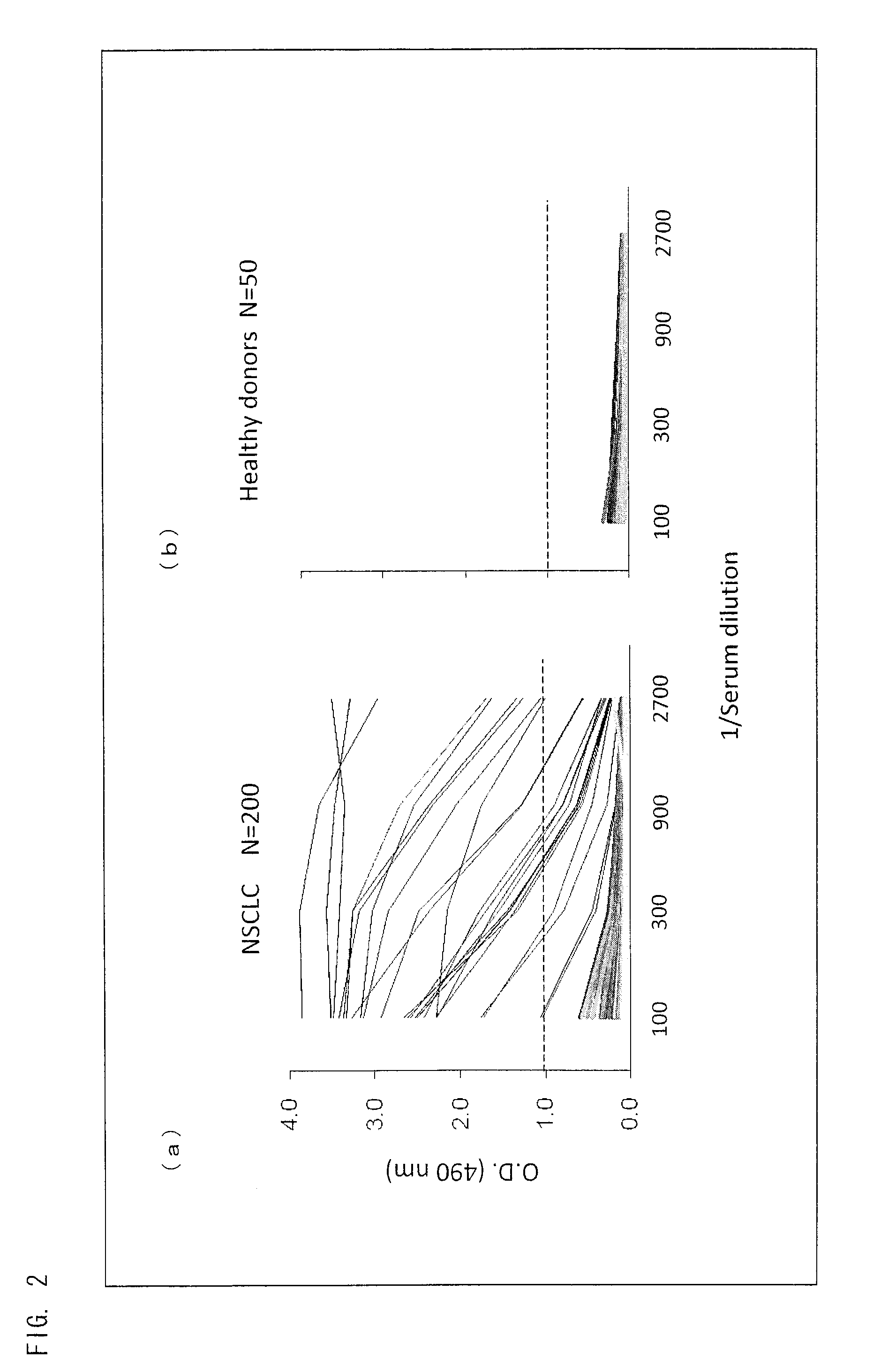
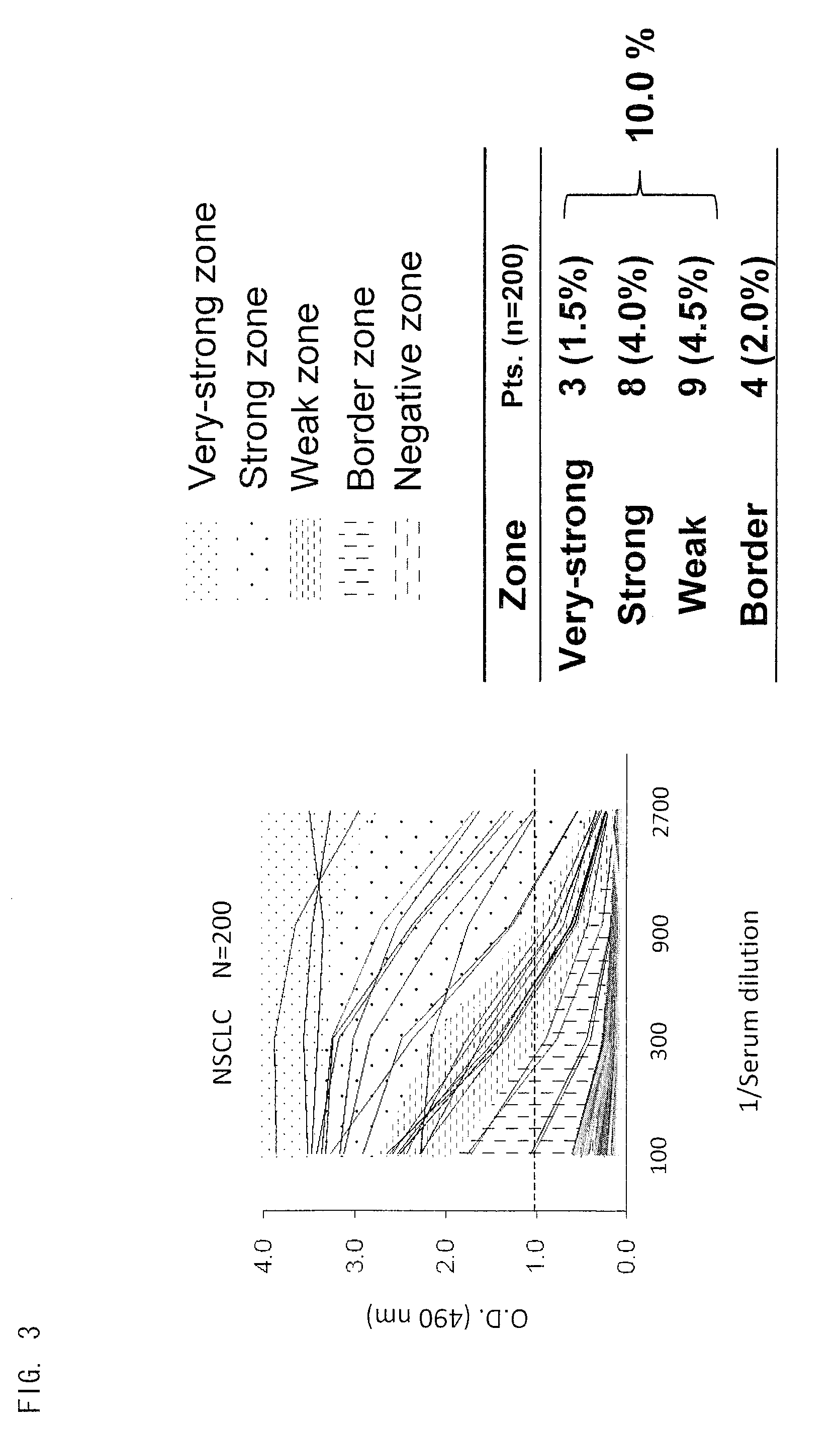
Peptide inducing xage-1b-specific immune reaction and utilization of same
InactiveUS20120064102A1Tumor rejection antigen precursorsPeptide/protein ingredientsHumoral immunityLung cancer
The object of the present invention is to clarify functions of XAGE-1b and to develop a vaccine therapy for cancer based on XAGE-1b. Humoral immunity or cellular immunity is induced against XAGE-1b in lung cancer, by use of a peptide comprising an amino acid sequence of any one of (a) through (e) or by use of a peptide comprising an amino acid sequence of any one of (f) through (k).
Owner:UNIV OKAYAMA
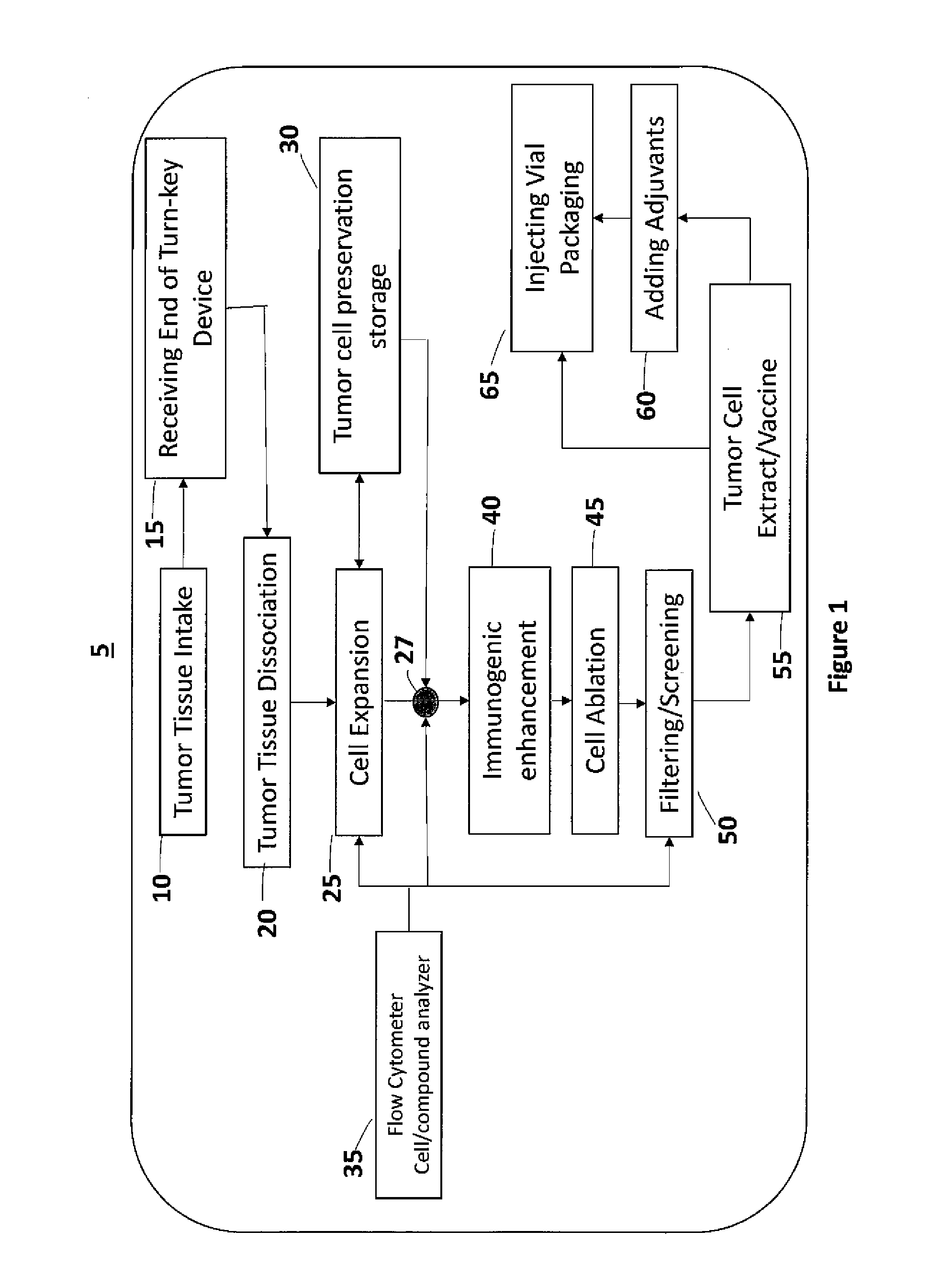
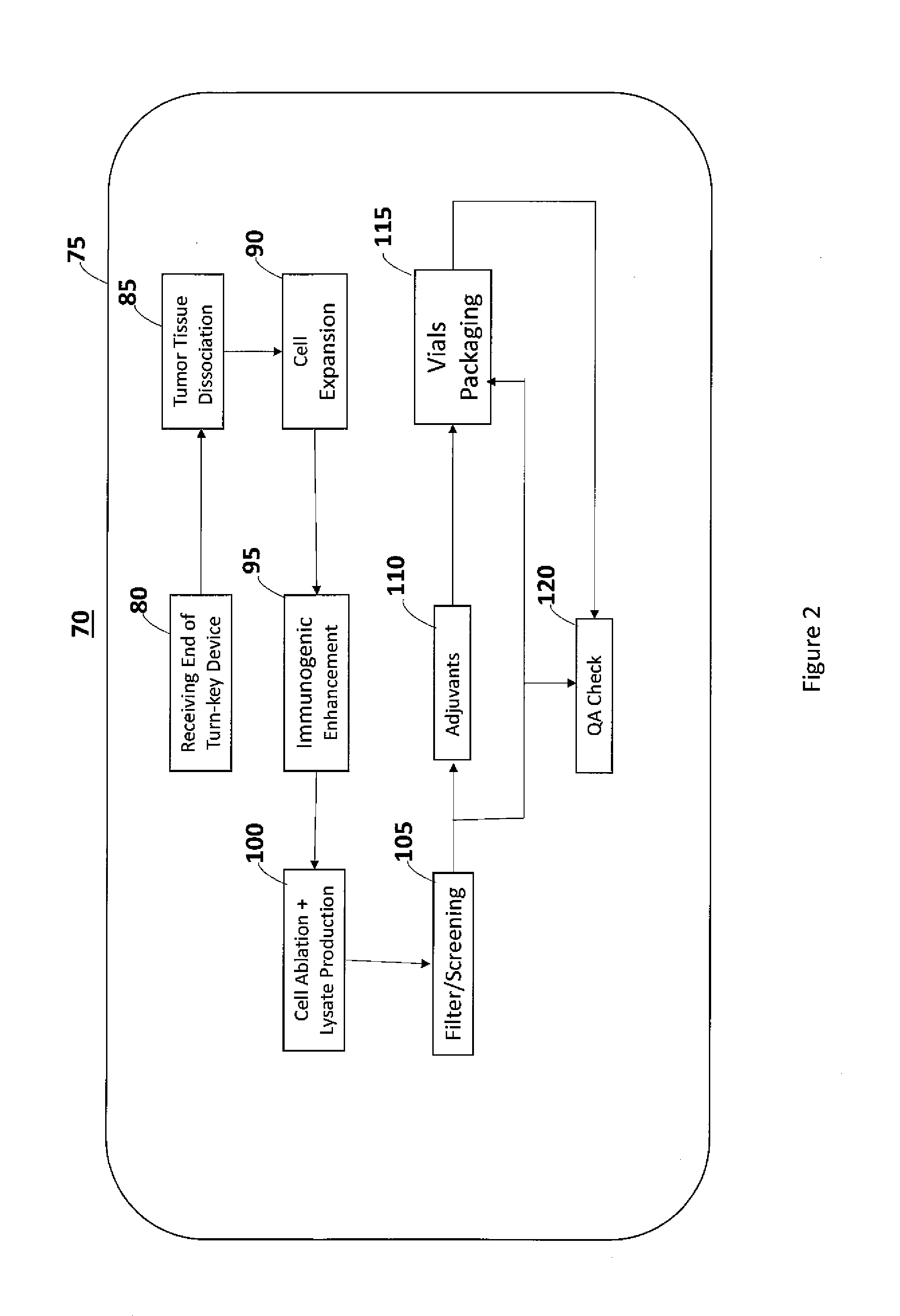
Self-Contained Device and System to Produce Ex-Vivo Autologous Whole Cell Tumor Vaccines
InactiveUS20160230139A1Reduce processing timeGood treatment effectBioreactor/fermenter combinationsBiological substance pretreatmentsSterile environmentAbnormal tissue growth
The invention disclosed herein aims to standardize and simplify the process of preparing Ex-Vivo autologous whole tumor cell vaccines. The present invention is a robust, stand-alone device and system for preparing autologous tumor cell vaccines in a completely self-contained sterile environment, and in a shortened time. This new device and system will process the extracted tumor with its associated stromal and endothelial cells into injectable tumor cell vaccines, administered automatically or semi-automatically. This invention incorporates a number of new biotechnologies to enhance therapeutic effects over other existing methods. This invention will allow a medical facility to prepare and administer autologous cancer cell vaccine therapy independently without having, or using, a GMP facility, while adhering to and maintaining GMP guidelines.
Owner:WU XIAODONG +1
Immunogenic compositions comprising a xenogenic prostate protein p501s
InactiveUS20060140965A1Enhancing induction of immunityBreaking the tolerance against the autologous antigenGenetic material ingredientsCancer antigen ingredientsHuman prostateImmunogenicity
The present invention relates to pharmaceutical / immunogenic compositions and methods for inducing an immune response against tumour-related antigens. More specifically, the invention relates to non-human prostate-specific antigens, more precisely to the non-human prostate-specific P501S, which can be used as xenogeneic antigen in prostate cancer vaccine therapy and as diagnostic agents for prostate tumours in humans, to immunogenic compositions containing them, to methods of manufacture of such compositions and to their use in medicine. Methods for formulating vaccines for immunotherapeutically treating P501S-expressing prostate tumors, prostatic hyperplasia, and prostate intraepithelilial neoplasia (PIN) are also provided.
Owner:GLAXO GROUP LTD +1
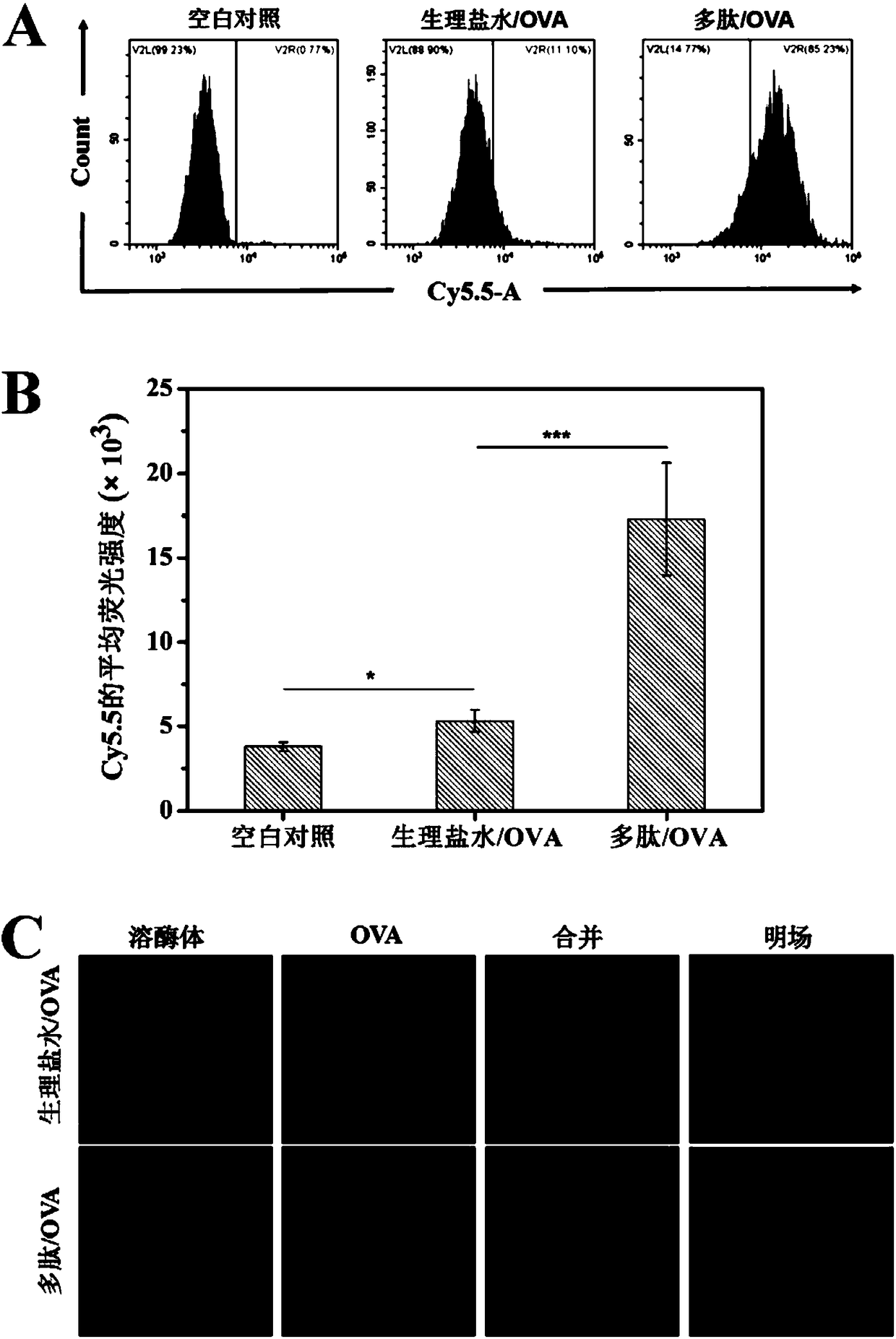
Methods for use of apoptotic cells to deliver antigen to dendritic cells for induction or tolerization of T cells
InactiveUS20060257431A1Enhancing antigen uptake and processingReduced activitySsRNA viruses negative-senseViral antigen ingredientsDendritic cellCytotoxicity
This invention relates to methods and compositions useful for delivering antigens to dendritic cells which are then useful for inducing antigen-specific cytotoxic T lymphocytes and T helper cells. This invention also provides assays for evaluating the activity of cytotoxic T lymphocytes. According to the invention, antigens are targeted to dendritic cells by apoptotic cells which may also be modified to express non-native antigens for presentation to the dendritic cells. The dendritic cells which are primed by the apoptotic cells are capable of processing and presenting the processed antigen and inducing cytotoxic T lymphocyte activity or may also be used in vaccine therapies.
Owner:THE ROCKEFELLER UNIV
Anti-malaria prime/boost vaccines
InactiveCN101068568AStrong immune responseAntiparasitic agentsImmunological disordersMalarial parasiteAdjuvant
Owner:JANSSEN VACCINES & PREVENTION BV +2
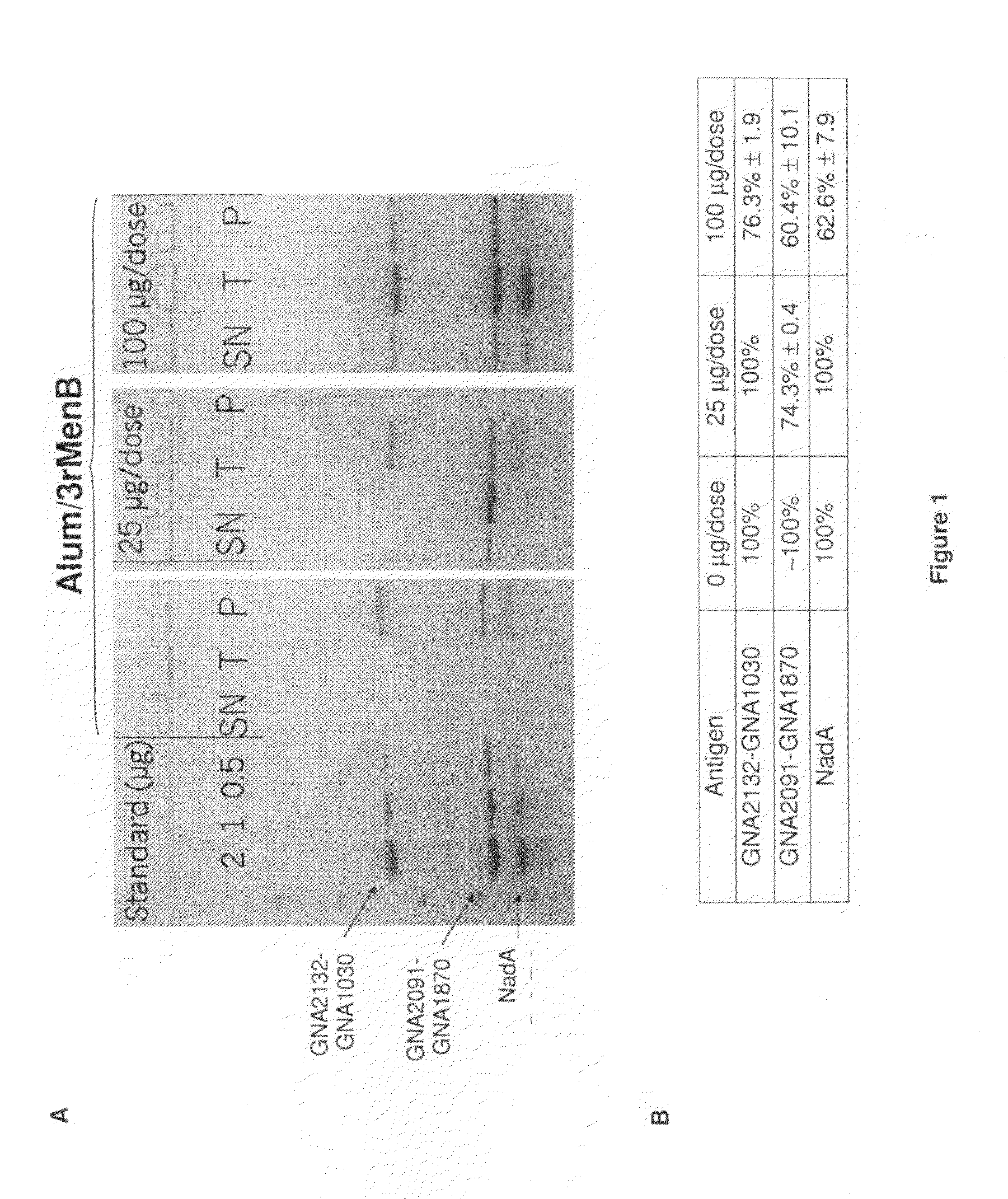
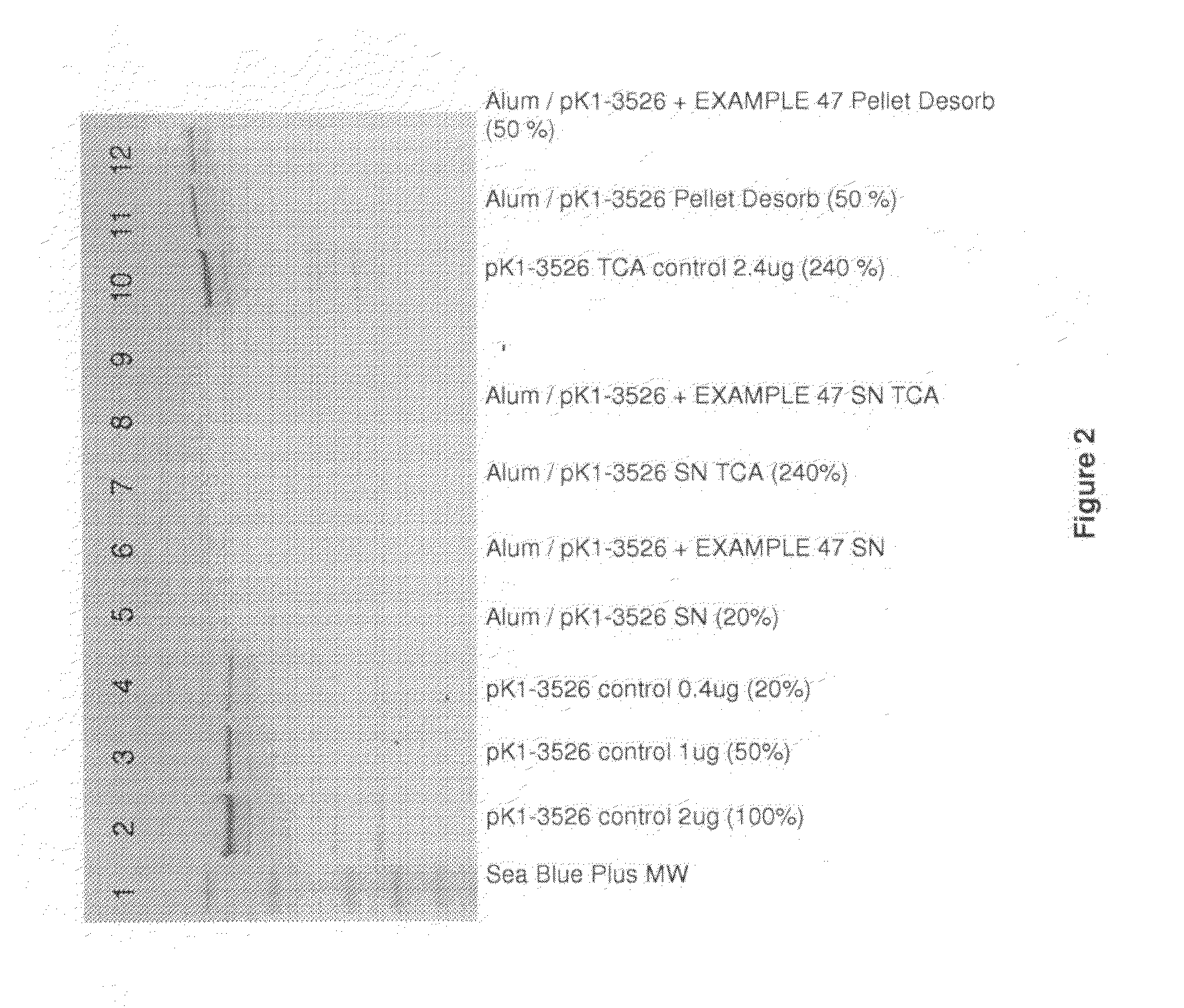
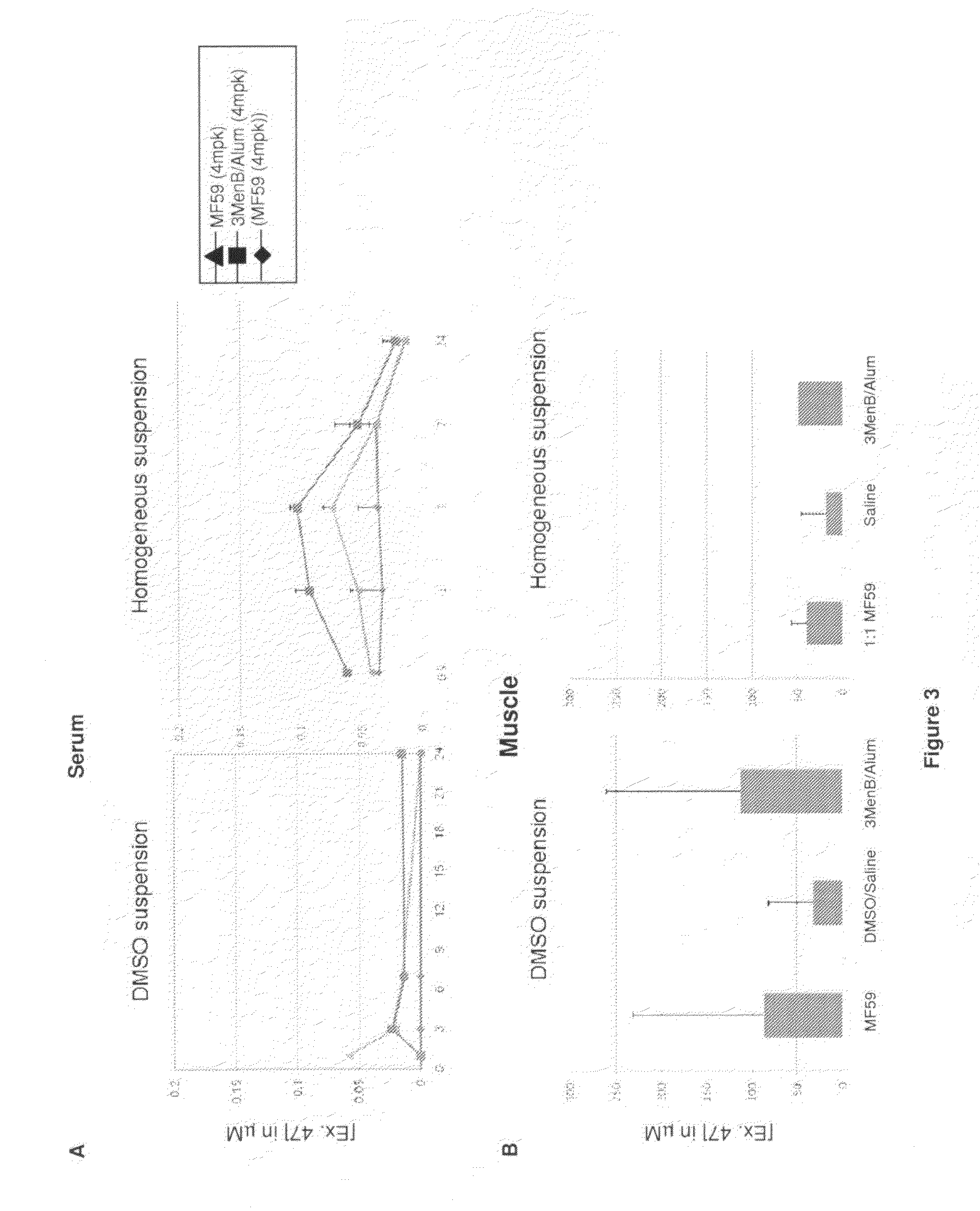
Homogenous suspension of immunopotentiating compounds and uses thereof
ActiveUS20130101629A1Improve efficiencySsRNA viruses negative-senseAntibacterial agentsAntigenAdjuvant
The present invention generally relates to homogeneous suspensions of small molecule immune potentiators (SMIPs) that are capable of stimulating or modulating an immune response in a subject in need thereof. The homogeneous suspensions may be used in combinations with various antigens or adjuvants for vaccine therapies.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Redox sensitive polypeptide based on cell-penetrating peptide and application of redox sensitive polypeptide in vaccine vector
ActiveCN108101966AQuick releaseOvercome the shortcomings of inability to induce strong antigen-specific cellular immunity in the bodyVertebrate antigen ingredientsDepsipeptidesCross-linkRedox responsive
The invention discloses redox sensitive polypeptide based on cell-penetrating peptide and application of the redox sensitive polypeptide in a vaccine vector. The sequence of the polypeptide is shown by SEQ ID NO:1. The polypeptide can be subjected to an electrostatic interaction with an antigen carrying a negative charge first, then cross-linking is performed with sulfydryl of cysteine, and the antigen is tightly wraped to form a stable polypeptide / antigen nano composite. In the nano composite, the sulfydryl of cysteine conducts spontaneous oxidation to form a disulfide bond, and the polypeptides are subjected to cross-linking to form a denser peptide / antigen condensation product. The redox sensitive polypeptide disclosed by the invention integrates the advantages of cell-penetrating peptide and the redox responding type disulfide bond cross-linking agent; by adopting new cell-penetrating peptide mediated redox responding type polypeptide as a vaccine adjuvant, the redox sensitive polypeptide overcomes the shortcoming that traditional immunologic adjuvant cannot induce an organism to generate strong antigen-specific cellular immunity, and is expected to be applied to clinical vaccine therapy.
Owner:JINAN UNIVERSITY
Methods for use of apoptotic cells to deliver antigen to dendritic cells for induction or tolerization of T cells
InactiveUS7129037B2Efficient deliveryEnhancing antigen uptake and processingVirusesGenetic material ingredientsDendritic cellT helper cell
This invention relates to methods and compositions useful for delivering antigens to dendritic cells which are then useful for inducing antigen-specific cytotoxic T lymphocytes and T helper cells. This invention also provides assays for evaluating the activity of cytotoxic T lymphocytes. According to the invention, antigens are targeted to dendritic cells by apoptotic cells which may also be modified to express non-native antigens for presentation to the dendritic cells. The dendritic cells which are primed by the apoptotic cells are capable of processing and presenting the processed antigen and inducing cytotoxic T lymphocyte activity or may also be used in vaccine therapies.
Owner:THE ROCKEFELLER UNIV
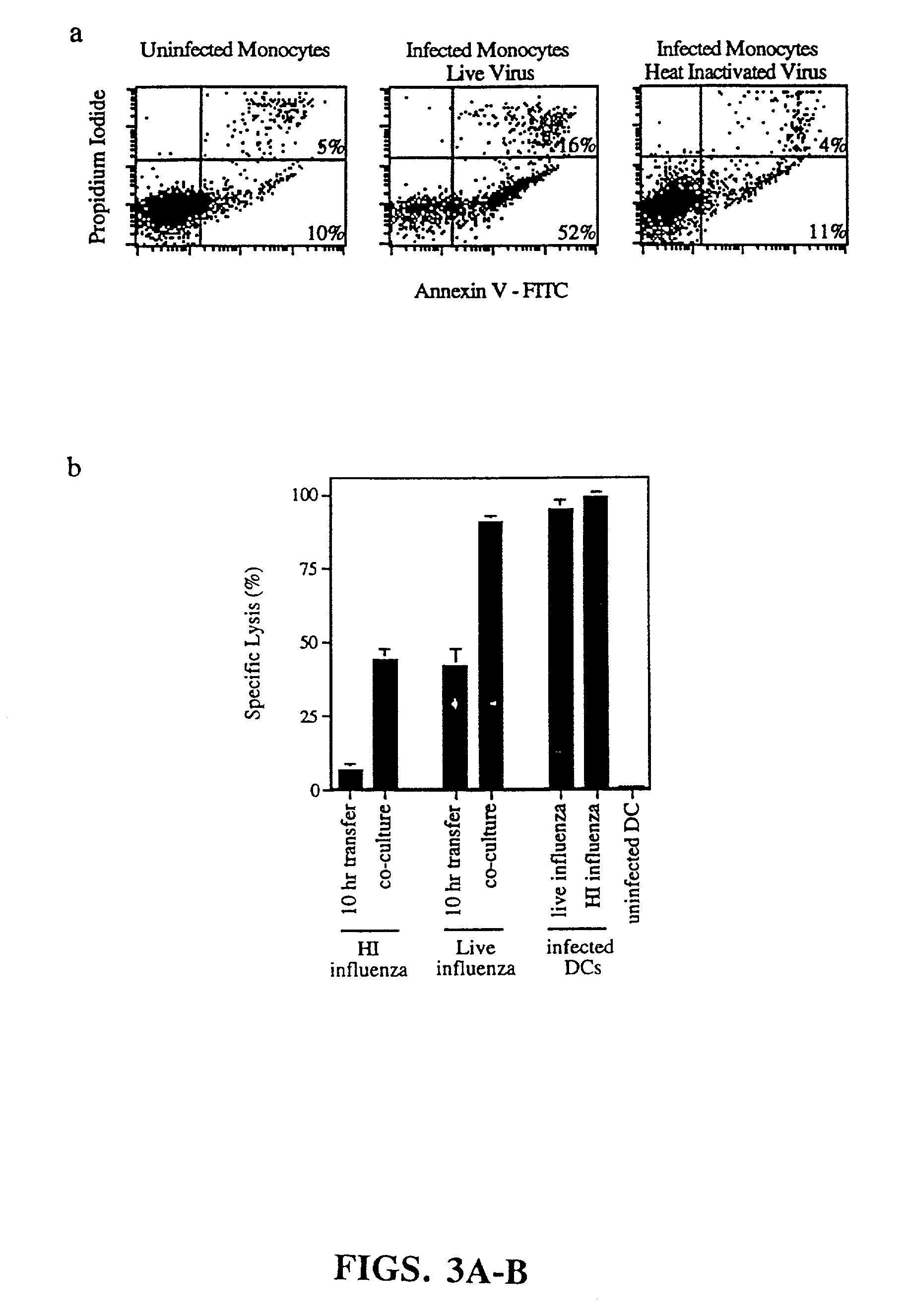
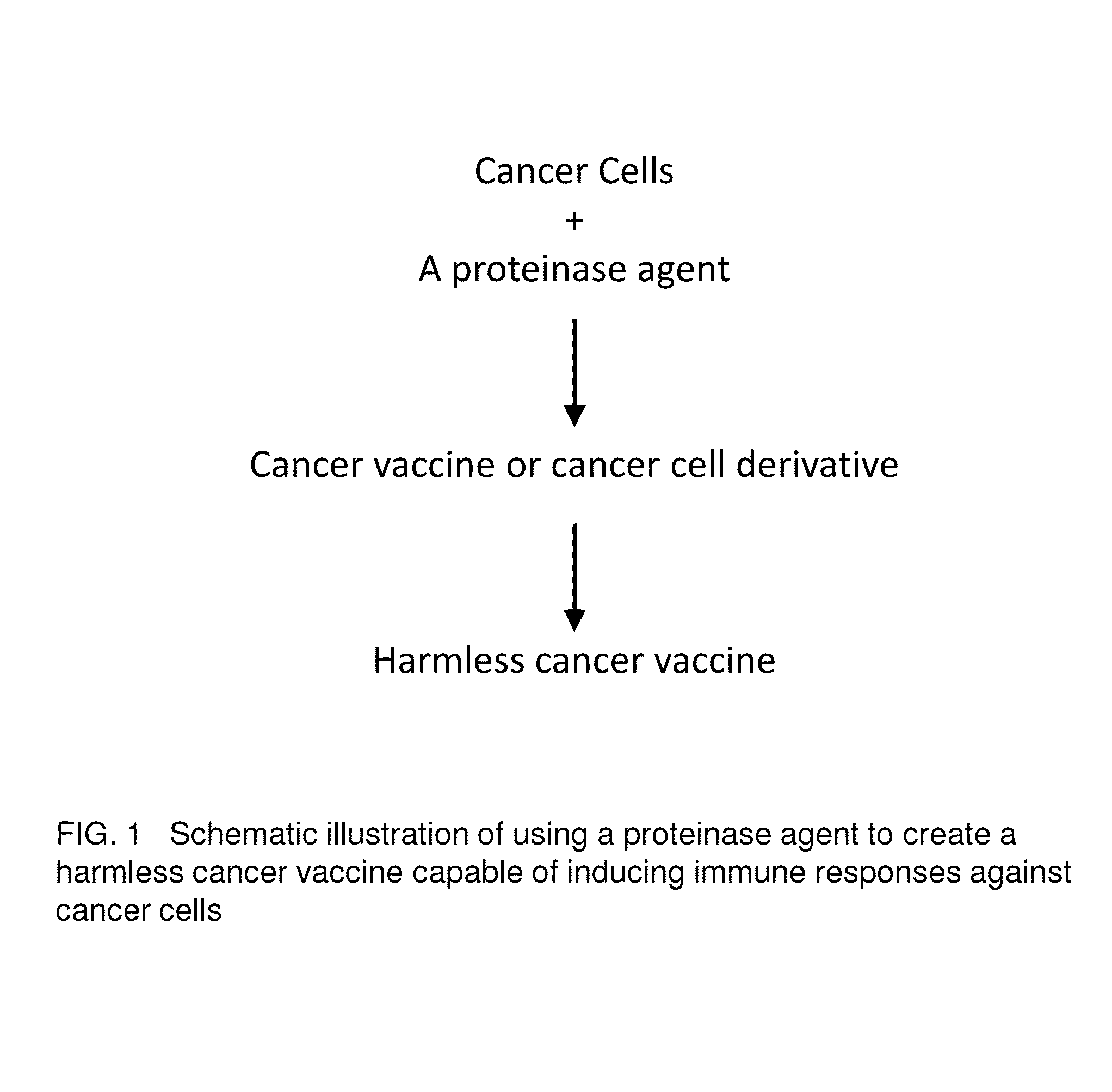
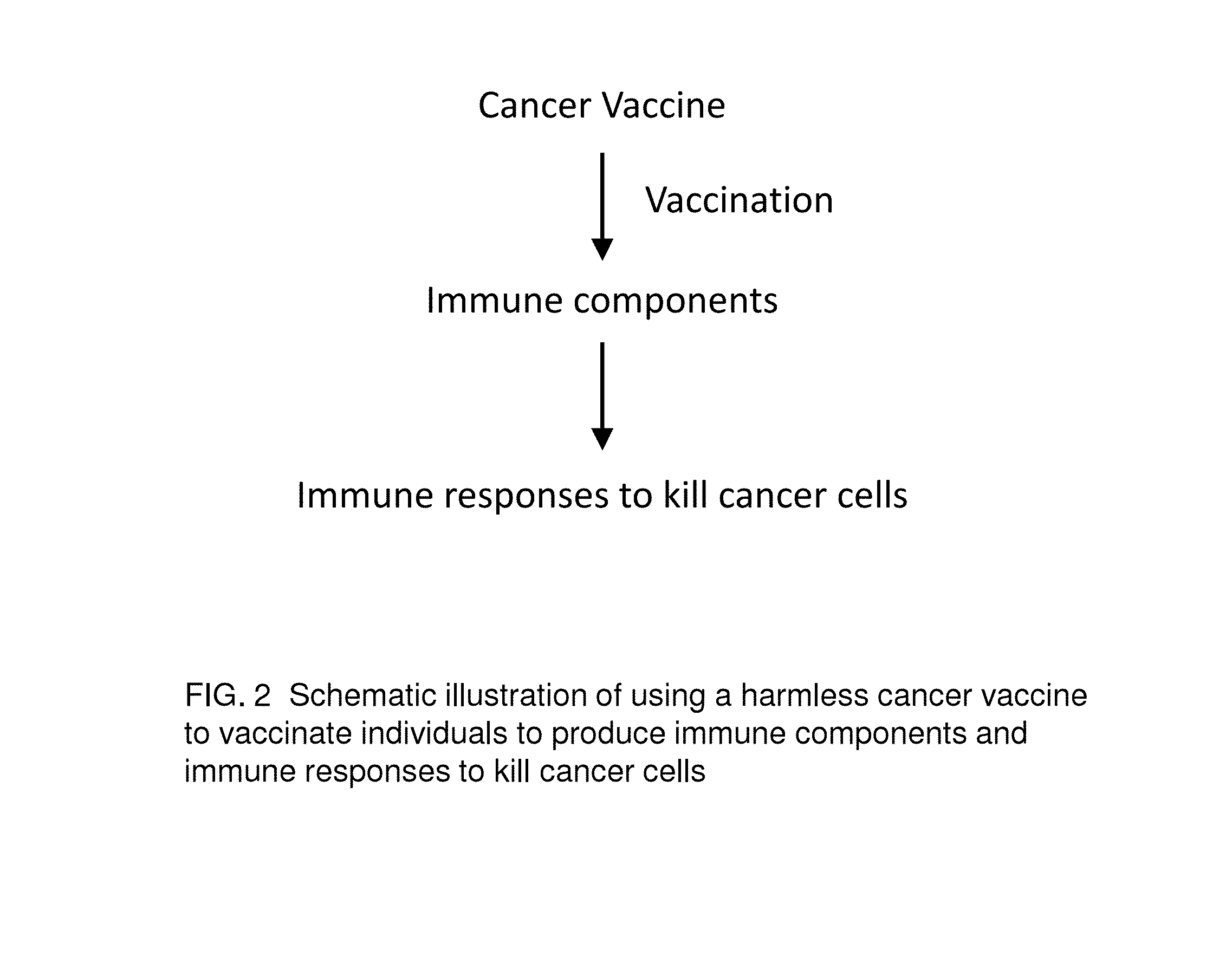
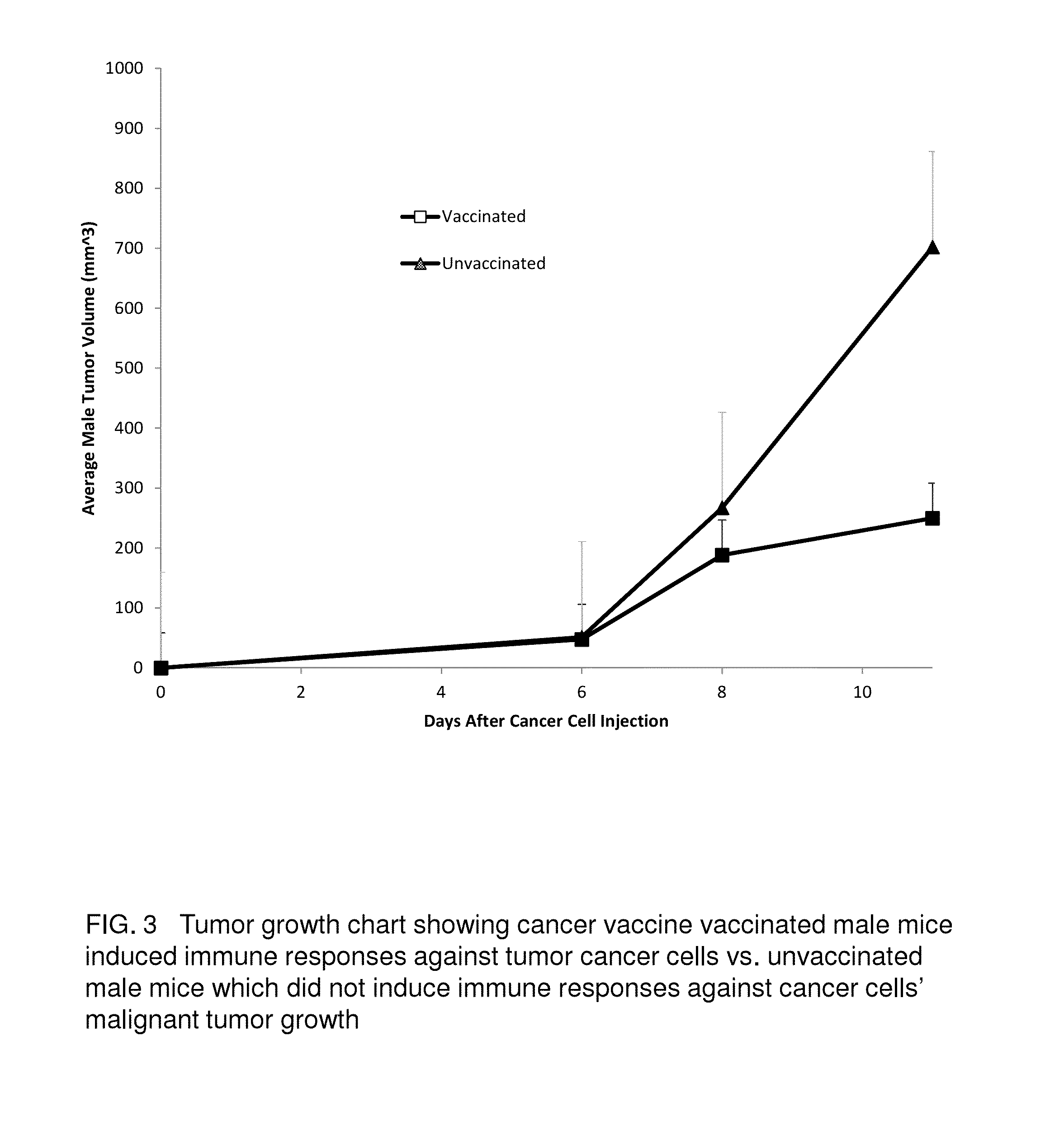
Proteinase-engineered Cancer Vaccine Induces Immune Responses to Prevent Cancer and to Systemically Kill Cancer Cells
The present invention provides a method of making a proteinase-engineered cancer vaccine for treating a cancer patient, especially for cancer patient at advanced / metastatic stage. The cancer vaccine comprises dead cancer cells with unbroken plasma membrane wherein the extracellular proteins and extracellular portion of membrane proteins are cleaved by proteinase digestion. The cancer vaccine may be derived from cancer cell lines or patients' cancer cells. The present invention provides a method of treating a cancer patient by administrating an effective amount of the cancer vaccine to the patient. In a clinical trial with 35 cancer patients, the cancer vaccine therapy brings cancer-free lives (no detectable tumor, micro tumor or cancer cells after treatment) back to 40% of these patients. The cancer vaccine therapy for the first time brings the promise of cure to this deadly disease. The present invention further provides a method of obtaining cancer-specific immune components from blood of individuals treated with the cancer vaccine.
Owner:QIAN YONG
Treatment and prevention of cancers associated with intracellular oncoproteins through antibody therapy or vaccination
We provide the application of intracellular antibody therapy to target intracellular oncoproteins or proteins to prevent cancer metastasis. We also provide the use of intracellular vaccine therapy comprising oncoprotein proteins for vaccination to stimulate the host to produce its own antibodies to prevent tumor formation. Antibodies can include chimeric antibodies.
Owner:AGENCY FOR SCI TECH & RES
Vaccine therapy for treatment of endometrial and ovarian cancer
ActiveUS20180256692A1Prevent relapseLow levelPharmaceutical delivery mechanismCancer antigen ingredientsGynecologyFolate-binding protein
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
A kind of quaternary phosphonium chitosan and its application as vaccine immune adjuvant
ActiveCN107200788BGood biocompatibilityEasy to synthesizeAntibody medical ingredientsAntiendomysial antibodiesPhosphonium
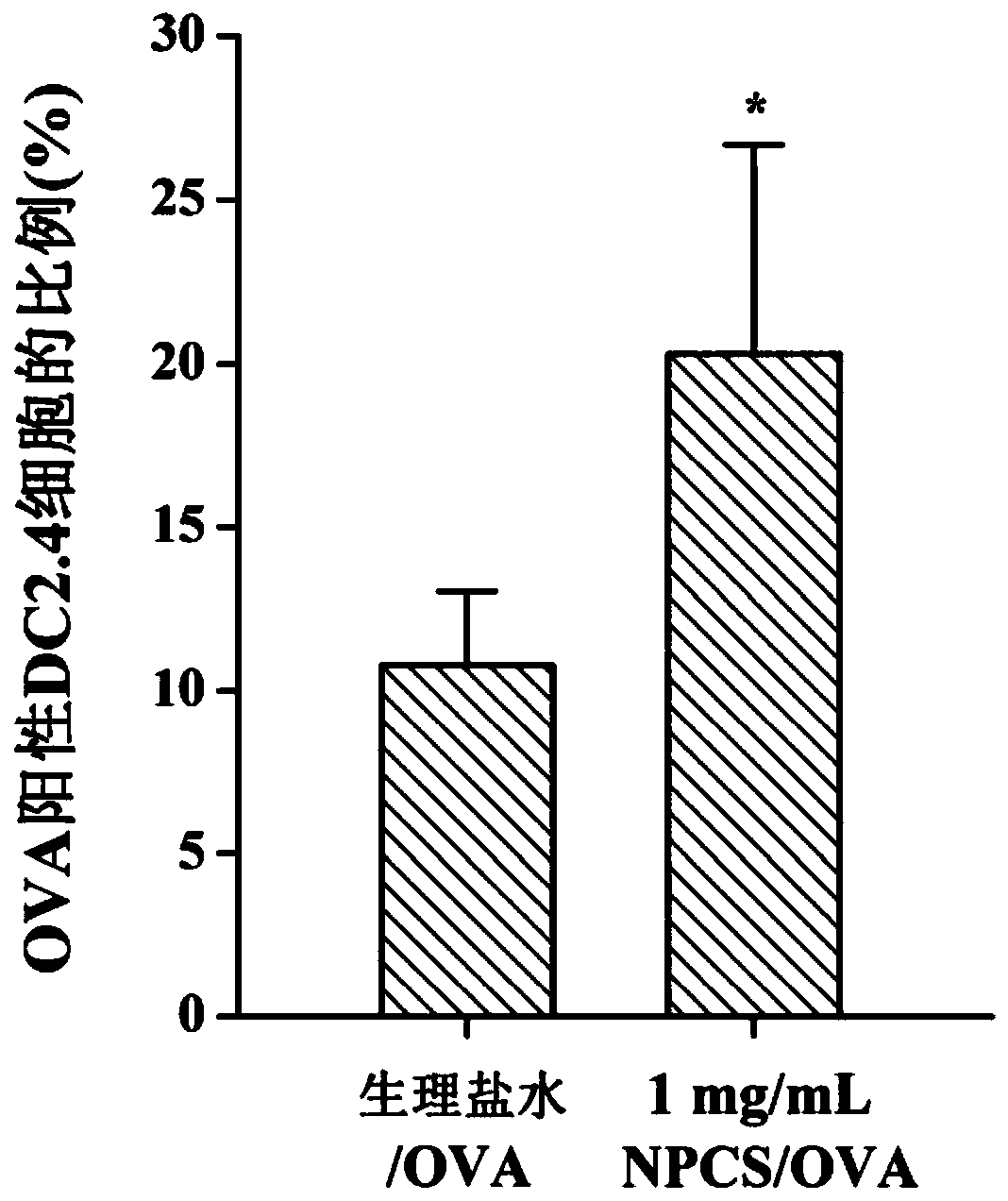
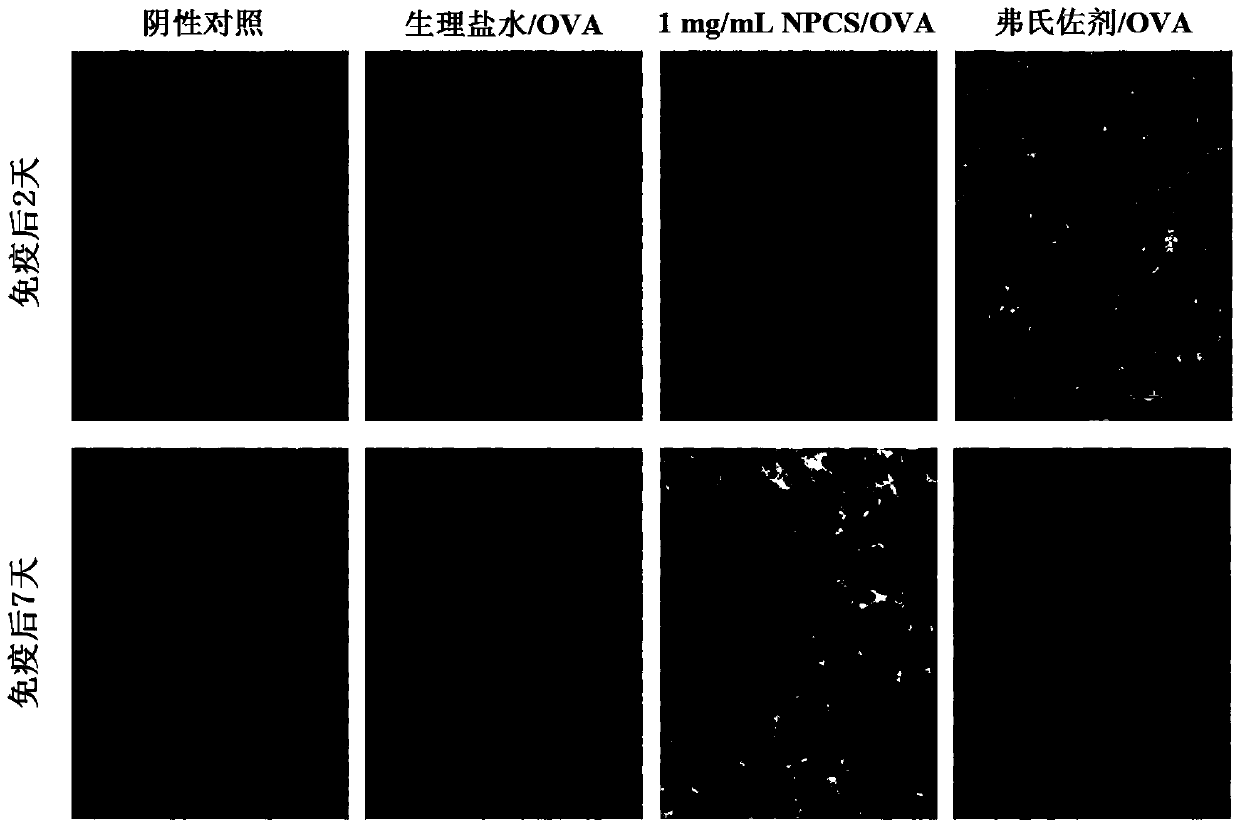
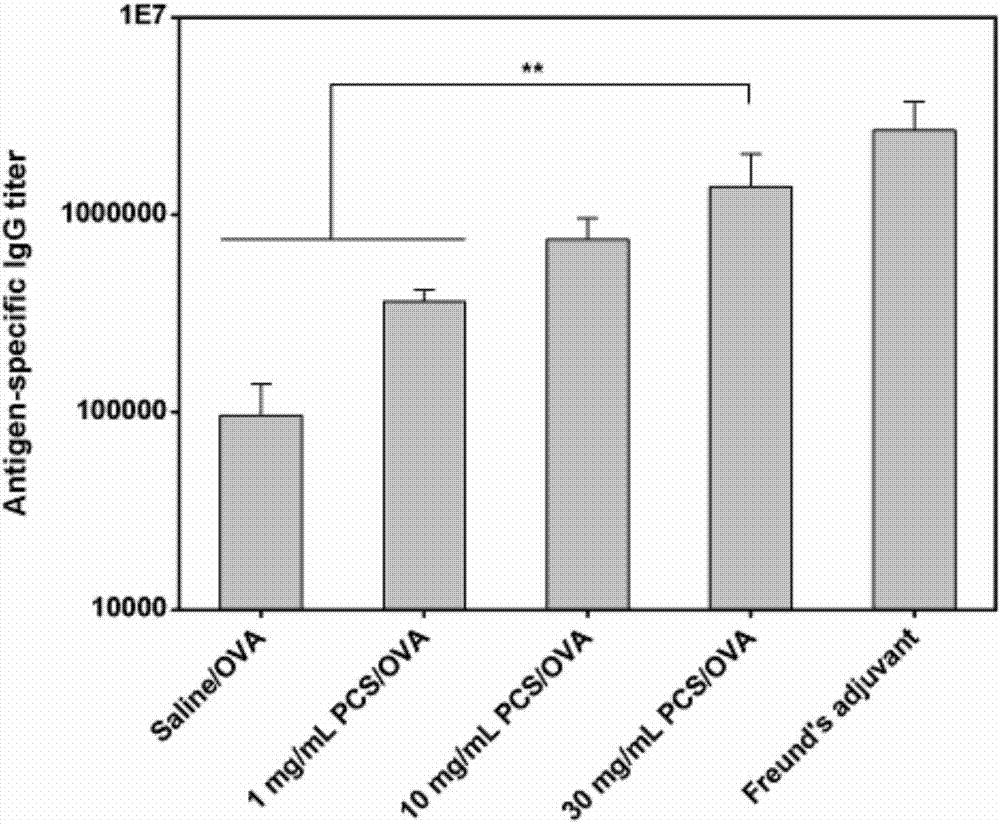
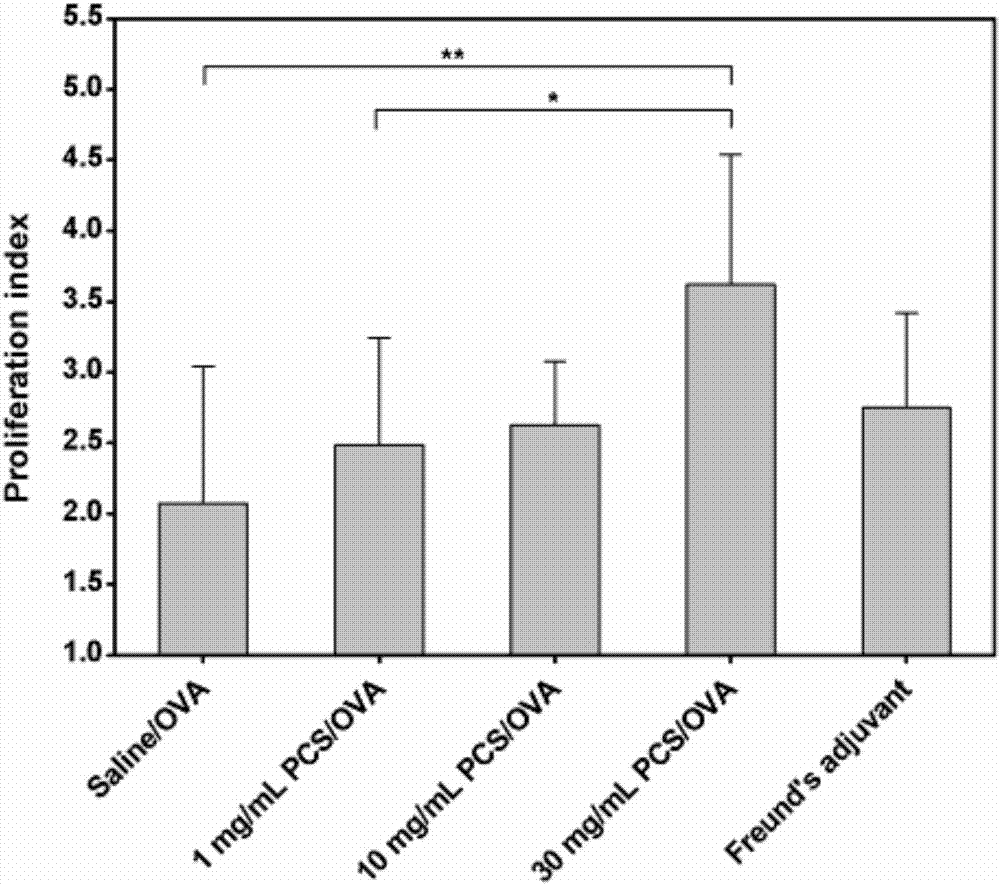
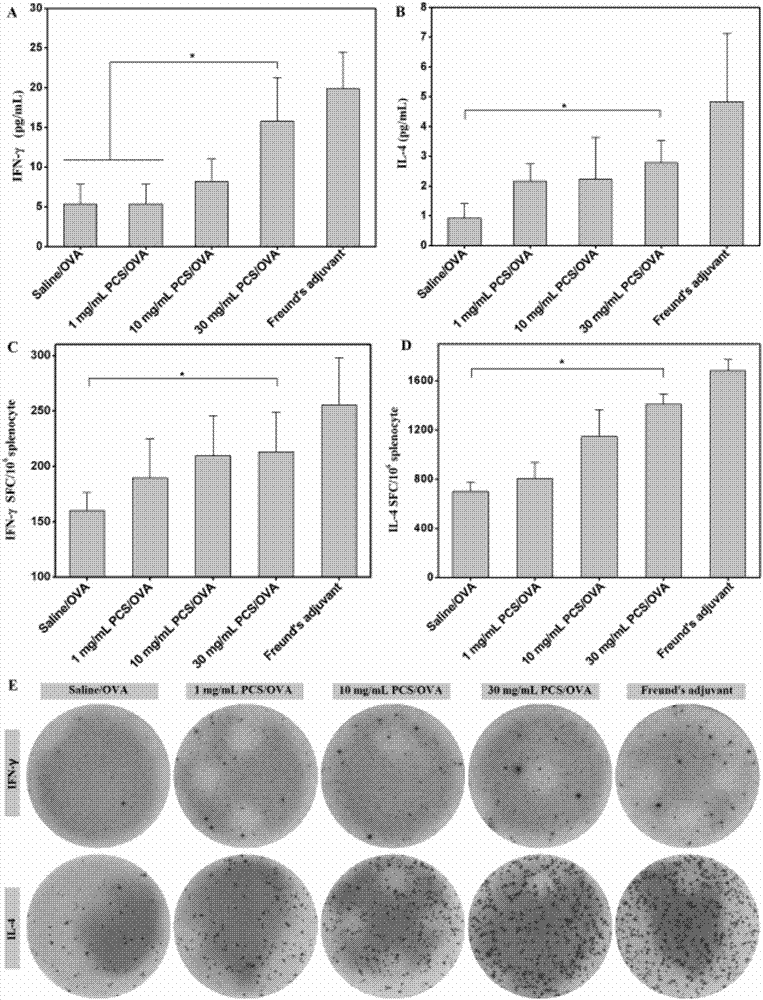
The invention discloses quaternary phosphonium chitosan and application of the quaternary phosphonium chitosan serving as a vaccine immune adjuvant. The preparation method of the quaternary phosphonium chitosan comprises the following steps: adding chitosan and 1-hydroxybenzotriazole into a solvent, so as to be subjected to ice bath reaction, and then adjusting the pH value to 4.8 to 5.5; then adding 3-hydroxypropyl triphenyl phosphorus bromide, and stirring the 3-hydroxypropyl triphenyl phosphorus bromide till the 3-hydroxypropyl triphenyl phosphorus bromide is completely dissolved; dissolving the 1-ethyl-(3-dimethylaminopropyl) carbodiie hydrochlide into the solvent, adding the 1-ethyl-(3-dimethylaminopropyl) carbodiie hydrochlide into a reaction system, precipitating, drying and dialyzing a product after reaction, and obtaining the quaternary phosphonium chitosan after freeze drying is performed. The quaternary phosphonium chitosan is taken as a vaccine immune adjuvant for the first time, and can promote the uptaking to antigen by DC2.4 cell, promote splenocyte proliferation, and induce higher levels of antigen-specific IgG antibody titer, IFN-gamma, IL-4 and IL-10, thereby having a significant application value in the field of vaccine therapy.
Owner:JINAN UNIVERSITY
Application of phosphorylated chitosan as immuno-adjuvant in vaccine therapy
InactiveCN106943592AInnovativeEasy to synthesizeAntibody medical ingredientsSolubilityDendritic cell
The invention discloses an application of phosphorylated chitosan as an immuno-adjuvant in vaccine therapy and belongs to the field of biomaterials and immuno-treatment. In the invention, for the first time, the phosphorylated chitosan serves as the immuno-adjuvant; compared with conventional immuno-adjuvants, the phosphorylated chitosan is easy to synthesize and is low in cost, and has excellent biodegradability and biocompatibility and has no damage on both organisms and environment. The phosphorylated chitosan is good in water-solubility and can be applied in in-vivo environment well without adverse toxic and side effects. The phosphorylated chitosan, as an immuno-adjuvant, can significantly induce higher-level antigen-specificity IgG antibody titer, promote immuno-reaction of IFN-gamma / IL-4 cell factor and CD4<+> / CD8<+> T lymphocytes and activate dendritic cells better, thereby enhancing immunogenicity of a vaccine and effectively improving antigen-specific immune response of organisms, so that the phosphorylated chitosan has important application value in the field of vaccine therapy.
Owner:JINAN UNIVERSITY
Vaccine for treatment or prevention of Burkholderia infection in a mammal
ActiveUS10328138B2Bacterial antigen ingredientsImmunoglobulins against bacteriaSequence IDMicrobiology
An agent for use in a vaccine therapy to prevent or treat a Burkholderia infection in a mammal, wherein the agent is selected from a polypeptide of SEQUENCE ID NO:1, or a therapeutically effective variant thereof having at least 90% sequence identity with SEQUENCE ID NO: 1; a polypeptide of SEQUENCE ID NO:3, or a therapeutically effective variant thereof having at least 90% sequence identity with SEQUENCE ID NO: 3; and an immunogenic portion of the polypeptides of SEQUENCE ID NO:1 or SEQUENCE ID NO:3.
Owner:UNIV COLLEGE DUBLIN NAT UNIV OF IRELAND DUBLIN
Mutated amyloid protein
InactiveCN101052718AProved metabolismOrganic active ingredientsNervous disorderAntigenAmyloidogenic Proteins
Owner:森启
Quaternary phosphonium chitosan and application of quaternary phosphonium chitosan serving as vaccine immune adjuvant
ActiveCN107200788AEasy to produceEfficient packagingAntibody medical ingredientsPhosphoniumFreeze-drying
The invention discloses quaternary phosphonium chitosan and application of the quaternary phosphonium chitosan serving as a vaccine immune adjuvant. The preparation method of the quaternary phosphonium chitosan comprises the following steps: adding chitosan and 1-hydroxybenzotriazole into a solvent, so as to be subjected to ice bath reaction, and then adjusting the pH value to 4.8 to 5.5; then adding 3-hydroxypropyl triphenyl phosphorus bromide, and stirring the 3-hydroxypropyl triphenyl phosphorus bromide till the 3-hydroxypropyl triphenyl phosphorus bromide is completely dissolved; dissolving the 1-ethyl-(3-dimethylaminopropyl) carbodiie hydrochlide into the solvent, adding the 1-ethyl-(3-dimethylaminopropyl) carbodiie hydrochlide into a reaction system, precipitating, drying and dialyzing a product after reaction, and obtaining the quaternary phosphonium chitosan after freeze drying is performed. The quaternary phosphonium chitosan is taken as a vaccine immune adjuvant for the first time, and can promote the uptaking to antigen by DC2.4 cell, promote splenocyte proliferation, and induce higher levels of antigen-specific IgG antibody titer, IFN-gamma, IL-4 and IL-10, thereby having a significant application value in the field of vaccine therapy.
Owner:JINAN UNIVERSITY
Method for concentration of virus
InactiveCN101983238AMaintain infectivitySimple and easy to concentrateBiological material analysisRecovery/purificationViral vectorInfectivity
Owner:JAPAN TOBACCO INC
Redox-sensitive polypeptides based on cell-penetrating peptides and their application in vaccine vectors
ActiveCN108101966BQuick releaseImprove immunityVertebrate antigen ingredientsDepsipeptidesDisulfide bondingPeptide antigen
The invention discloses redox sensitive polypeptide based on cell-penetrating peptide and application of the redox sensitive polypeptide in a vaccine vector. The sequence of the polypeptide is shown by SEQ ID NO:1. The polypeptide can be subjected to an electrostatic interaction with an antigen carrying a negative charge first, then cross-linking is performed with sulfydryl of cysteine, and the antigen is tightly wraped to form a stable polypeptide / antigen nano composite. In the nano composite, the sulfydryl of cysteine conducts spontaneous oxidation to form a disulfide bond, and the polypeptides are subjected to cross-linking to form a denser peptide / antigen condensation product. The redox sensitive polypeptide disclosed by the invention integrates the advantages of cell-penetrating peptide and the redox responding type disulfide bond cross-linking agent; by adopting new cell-penetrating peptide mediated redox responding type polypeptide as a vaccine adjuvant, the redox sensitive polypeptide overcomes the shortcoming that traditional immunologic adjuvant cannot induce an organism to generate strong antigen-specific cellular immunity, and is expected to be applied to clinical vaccine therapy.
Owner:JINAN UNIVERSITY
Homogenous suspension of immunopotentiating compounds and uses thereof
The present invention generally relates to homogeneous suspensions of small molecule immune potentiators (SMIPs) that are capable of stimulating or modulating an immune response in a subject in need thereof. The homogeneous suspensions may be used in combinations with various antigens or adjuvants for vaccine therapies.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Vaccine therapy for treatment of endometrial and ovarian cancer
ActiveUS11338025B2Prevent relapseLow levelPharmaceutical delivery mechanismCancer antigen ingredientsEndometrial CarcinomasFolate-binding protein
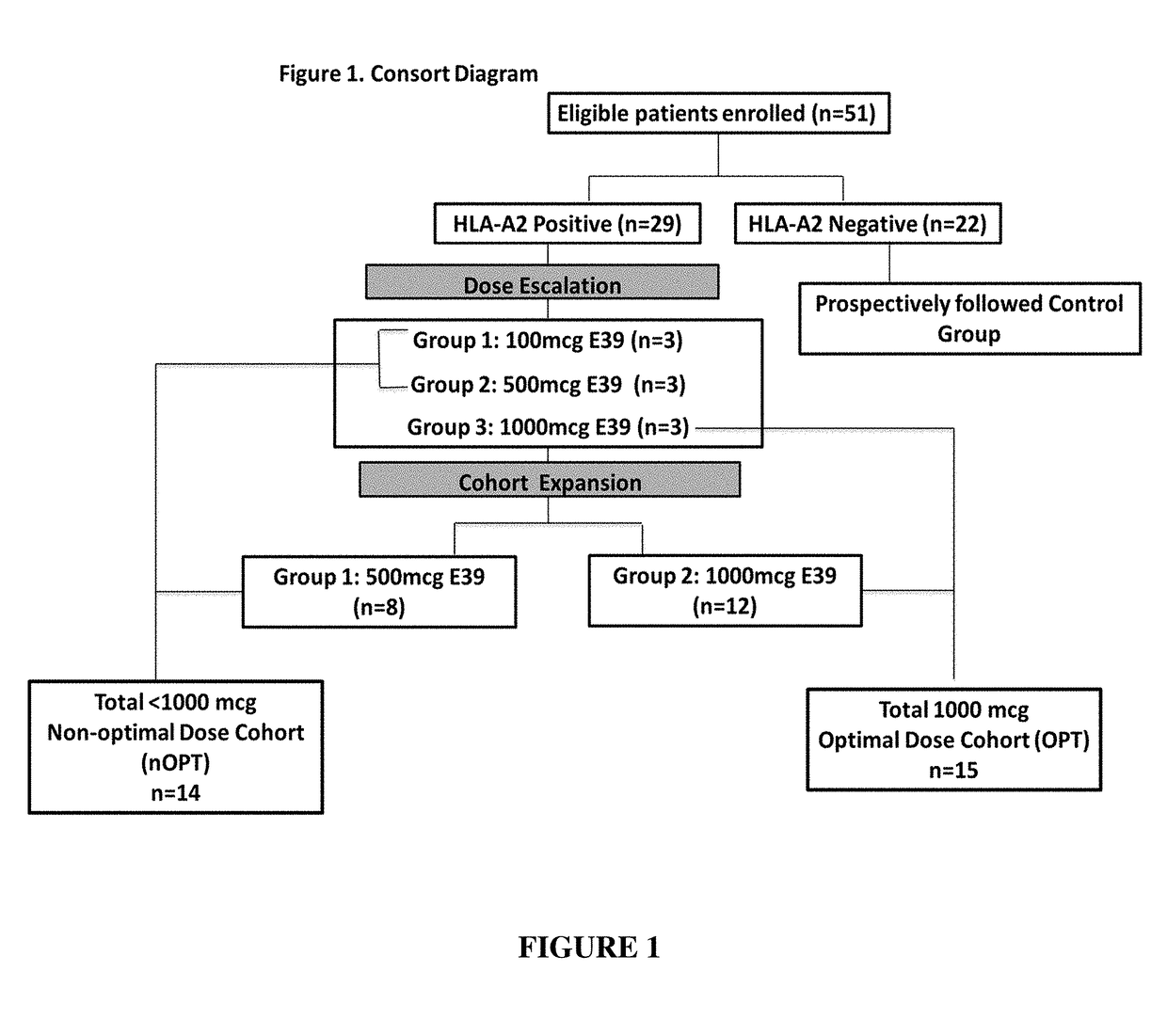
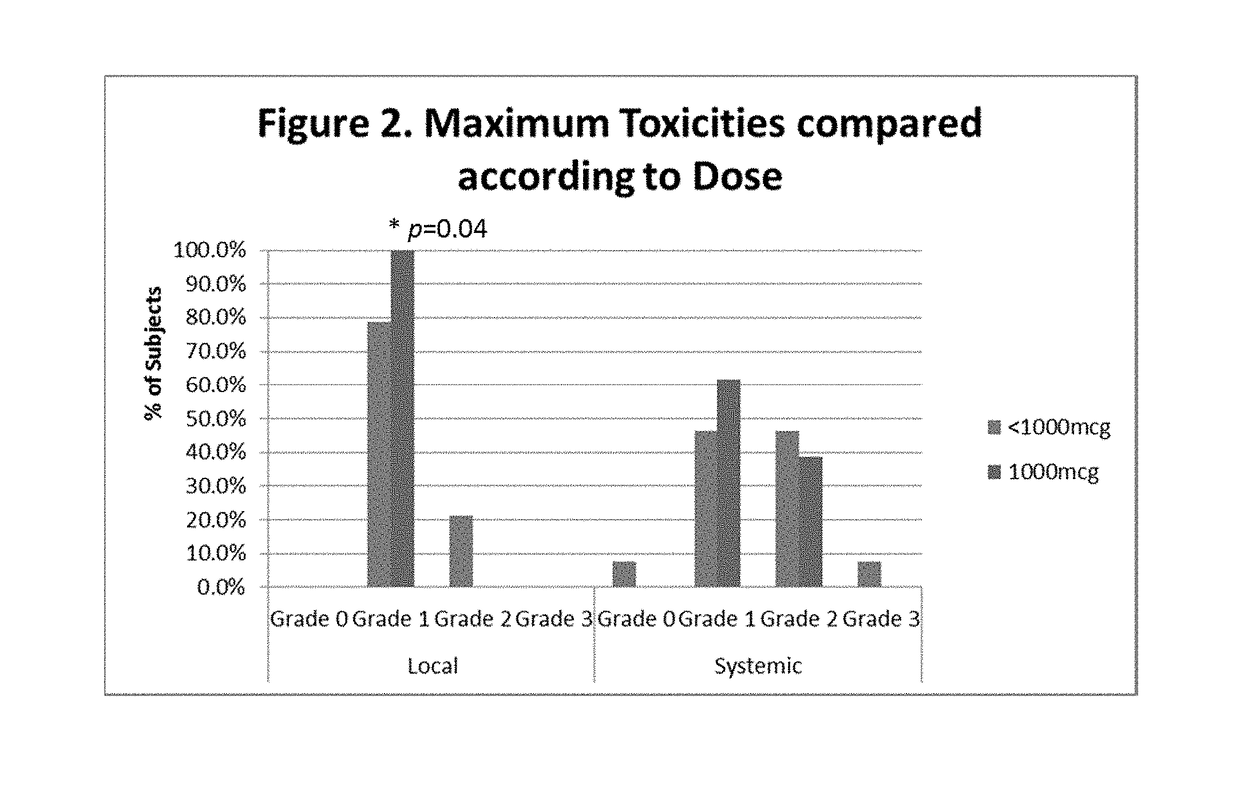
The present invention provides methods of preventing or reducing the risk of recurrence of endometrial and ovarian cancers which express low levels of folate binding protein (FBP) by administration of a vaccine containing an E39 peptide.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com