Anti-malaria prime/boost vaccines
A malaria and antigen technology, applied in the medical field, can solve the problems of insufficient protection and insufficient immunogenicity
Inactive Publication Date: 2007-11-07
JANSSEN VACCINES & PREVENTION BV +2
View PDF37 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Other vaccine candidates tested were either insufficiently immunogenic or immunogenic but insufficiently protective
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
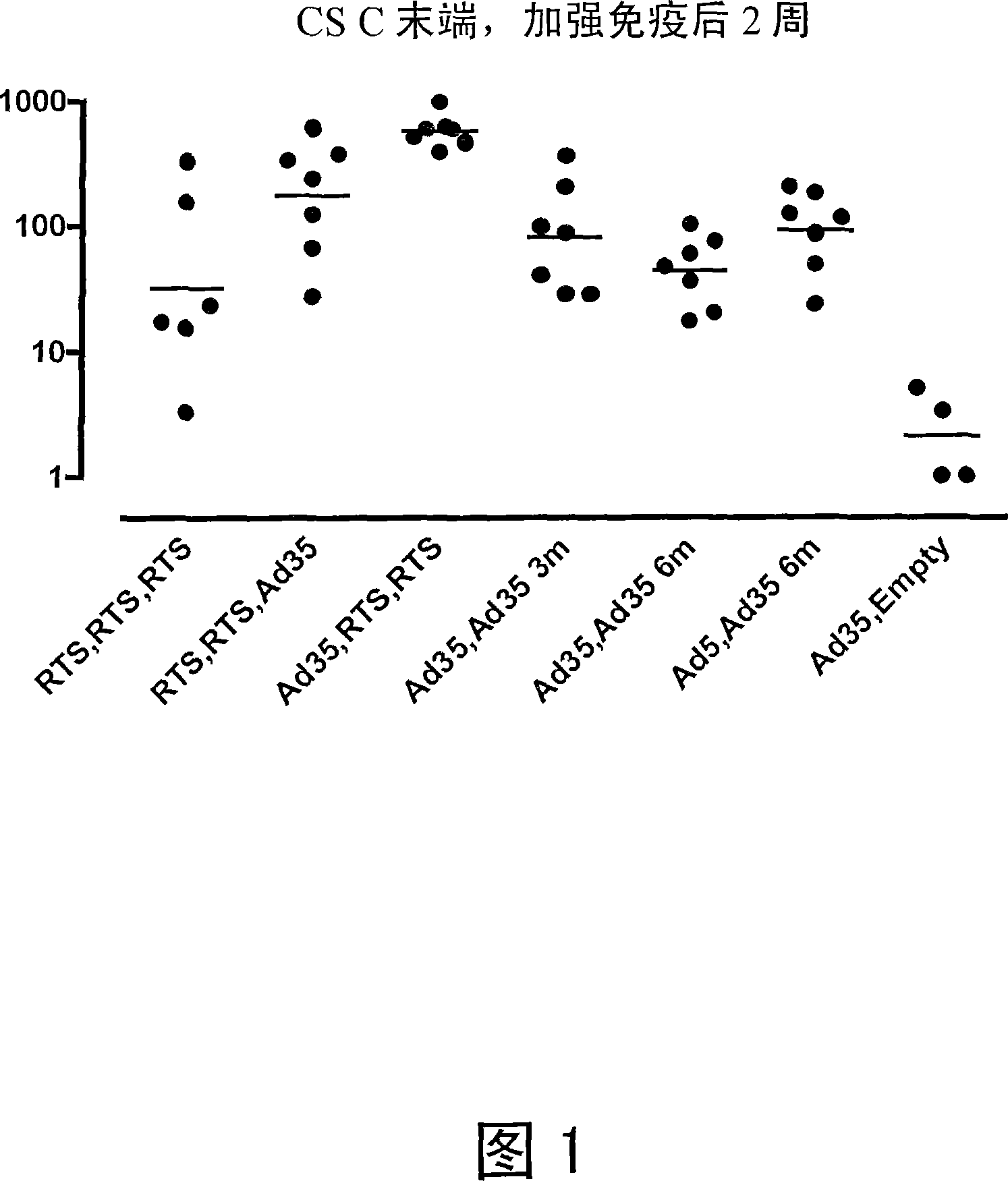
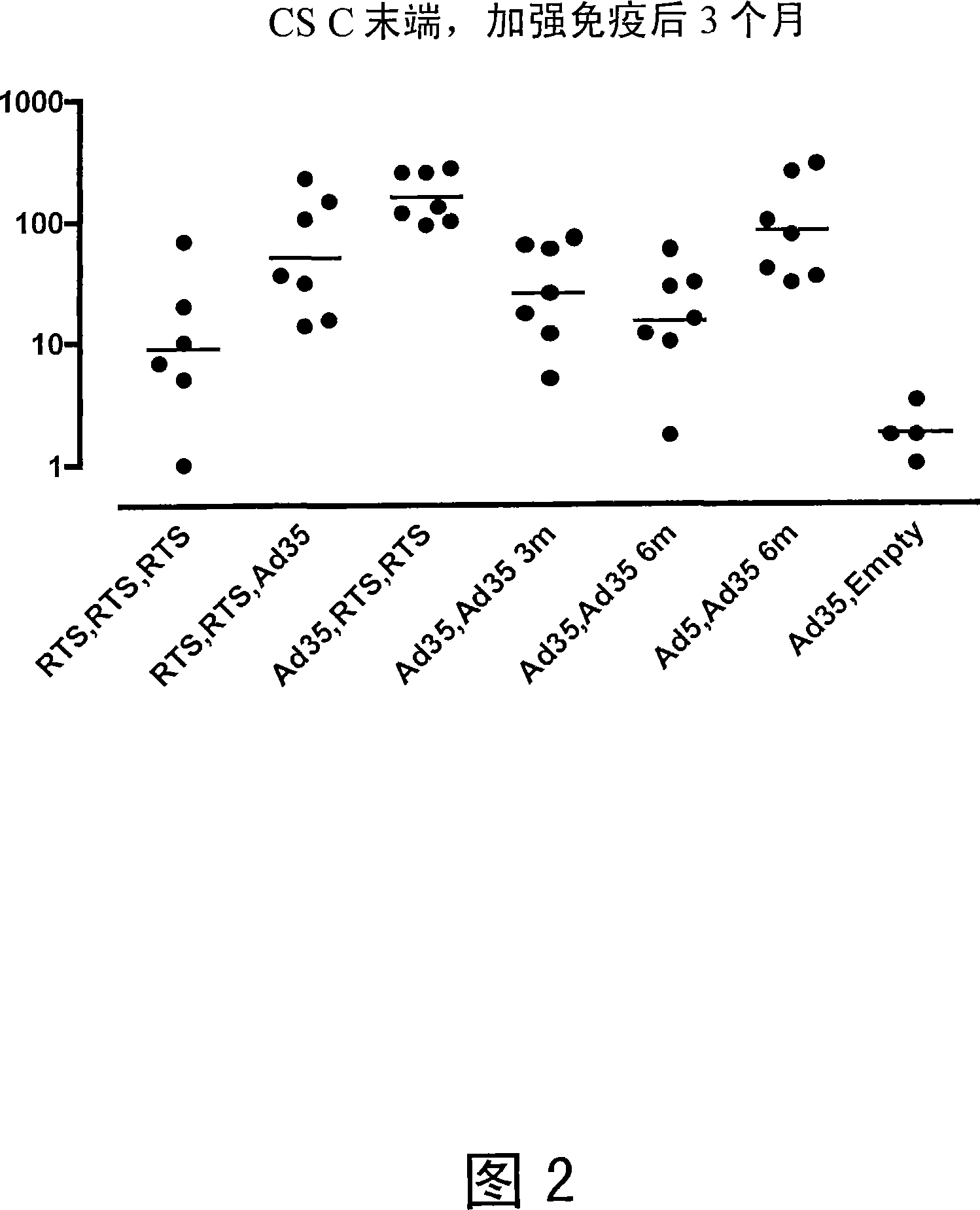
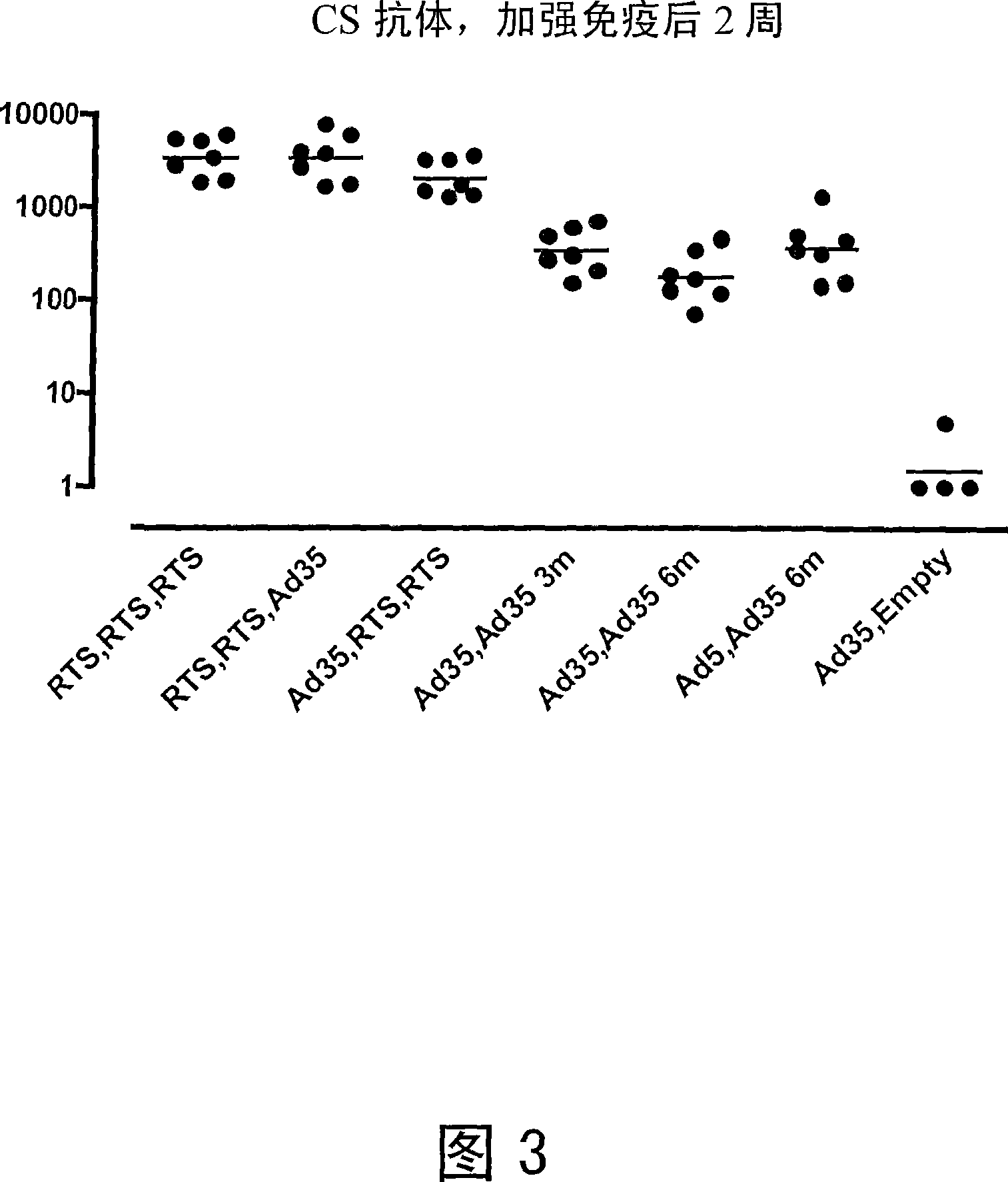
[0100] Heterologous Prime / Boost Immunization of Rhesus Monkeys Using Recombinant Adenoviral Vectors and Purified Adjuvanted Proteins
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Login to View More
Abstract
The invention relates to novel vaccine regimens in which specific prime / boost regimens are applied using low-neutralized recombinant adenoviral vectors harboring nucleic acids encoding antigens from Plasmodium falciparum and purified recombinant protein vaccines such as RTS,S, in the context of appropriate adjuvants.
Description
technical field [0001] The present invention relates to the field of medicine. Specifically, the present invention relates to a novel prime / boost vaccine strategy for preventing falciparum malaria using recombinantly produced adenovirus vectors and purified proteins combined with adjuvants. Background technique [0002] Malaria is currently one of the most prevalent infectious diseases in tropical and subtropical regions of the world. Each year, malaria infection kills an estimated 2.7 million people in developing countries. The widespread prevalence and increased incidence of malaria is due to the increase in the number of drug-resistant parasites and insecticide-resistant parasite vectors. Other factors include environmental and climate change, national instability, and increased population mobility. [0003] Malaria is caused by mosquito-borne blood-borne protozoan parasites belonging to the genus Plasmodium. Four species of Plasmodium (Plasmodium falciparum, P.vivax,...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K39/015
CPCA61K2039/55572A61K2039/5256A61K2039/55577A61K39/015A61K2039/6075A61P33/06A61P37/02A61P43/00Y02A50/30
Inventor 马里亚·格拉西亚·帕乌杰普·古德斯米特约瑟夫·D·科恩帕特里斯·M·杜波依斯V·安·斯图尔特唐纳德·海普纳尔
Owner JANSSEN VACCINES & PREVENTION BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com