A kind of quaternary phosphonium chitosan and its application as vaccine immune adjuvant
A technology of phosphonium chitosan and immune adjuvant, which is applied in the field of biomedical materials, can solve the problems of poor water solubility of derivatives, and achieve the effects of good biological safety, simple preparation, and improved utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A kind of quaternary phosphonium chitosan is prepared by the following steps:
[0039] Add 400mg chitosan (CS) and 664mg HOBt to a 250mL three-necked flask, and then add 30mL H 2 The mixture of O / DMSO (v / v=2 / 1) was stirred for 2.5 h in ice bath. The pH was then adjusted to 4.8-5.5. Add 1.052g of CTPB (3-carboxypropyltriphenylphosphonium bromide) and stir until completely dissolved. Dissolve 940mg EDC·HCl in 10mL H 2 A mixture of O / DMSO (v / v=1 / 1) was added to the flask and stirred for 24 hours. The product was precipitated with a mixture of acetone / ether (v / v=2 / 1), and then the precipitated product was vacuum-dried for 24 hours, dissolved in ultrapure water, dialyzed in ultrapure water for 3 days, and quaternary was obtained after freeze-drying. Phosphonium chitosan (NPCS).
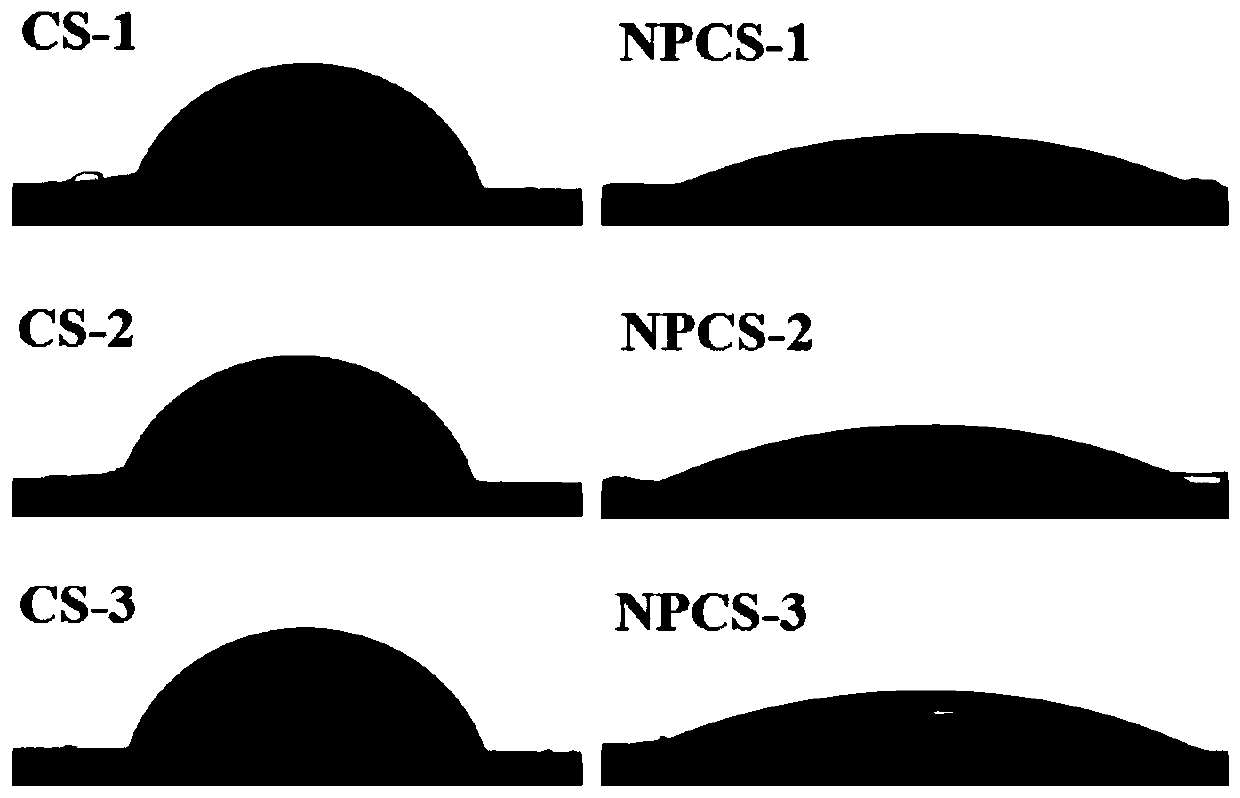
[0040] The contact angle experiment of chitosan (CS) and the quaternary phosphonium chitosan (NPCS) that the present embodiment makes: Chitosan (CS) and the quaternary phosphonium chitosan (NPC...
Embodiment 2
[0043] The application of above-mentioned quaternary phosphonium chitosan as vaccine immune adjuvant:
[0044] 1. Preparation of vaccine preparations
[0045] With ovalbumin OVA as the model antigen, vaccines of different formulations were prepared according to Table 1, and then intramuscularly injected into mice.
[0046] Table 1 Vaccine formulations for intramuscular injection
[0047]
[0048] 2. Mouse immunization scheme
[0049] Intramuscular injection: female Balb / c mice at 6-8 weeks were randomly divided into 6 groups, 5 mice in each group; 100 μL of vaccine solution (each injection containing 50 μg OVA, each leg inject half the dose). Ten days after the third immunization, the blood was collected, the serum was separated, and stored at -20°C for later use; the spleen of the mice was taken, ground, and resuspended to prepare spleen cell suspensions of different concentrations for later use.
[0050] 3. Determination of various immune indicators
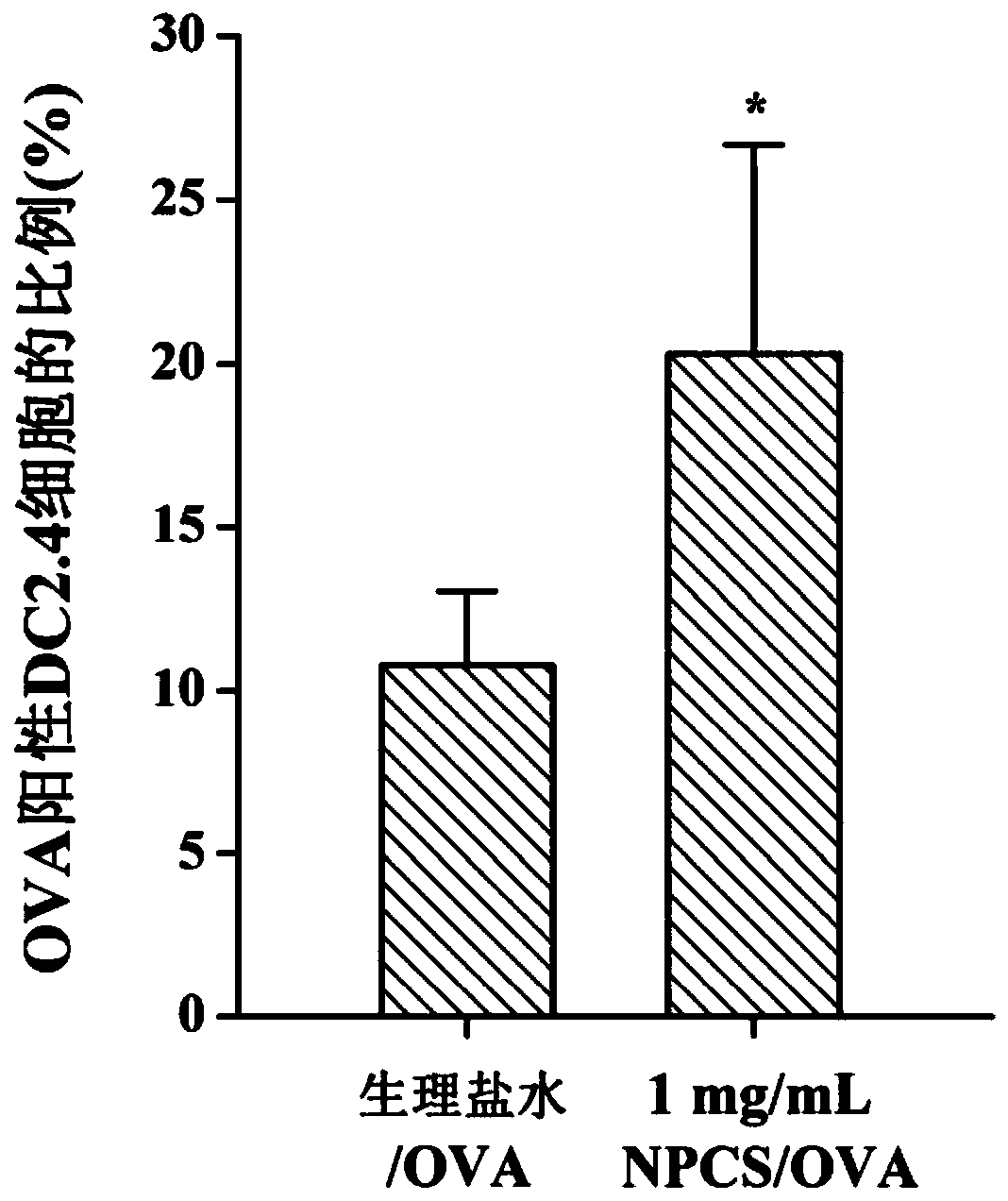
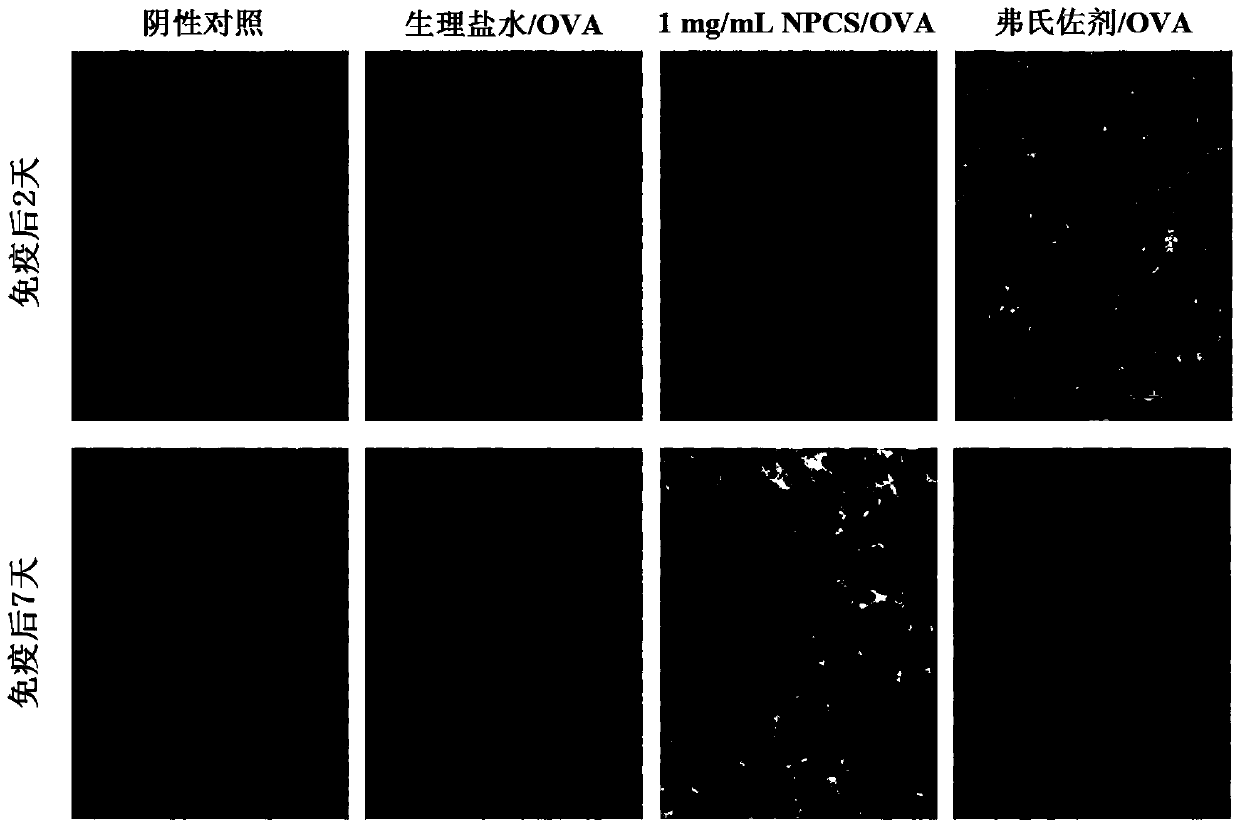
[0051] 1. Uptak...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com