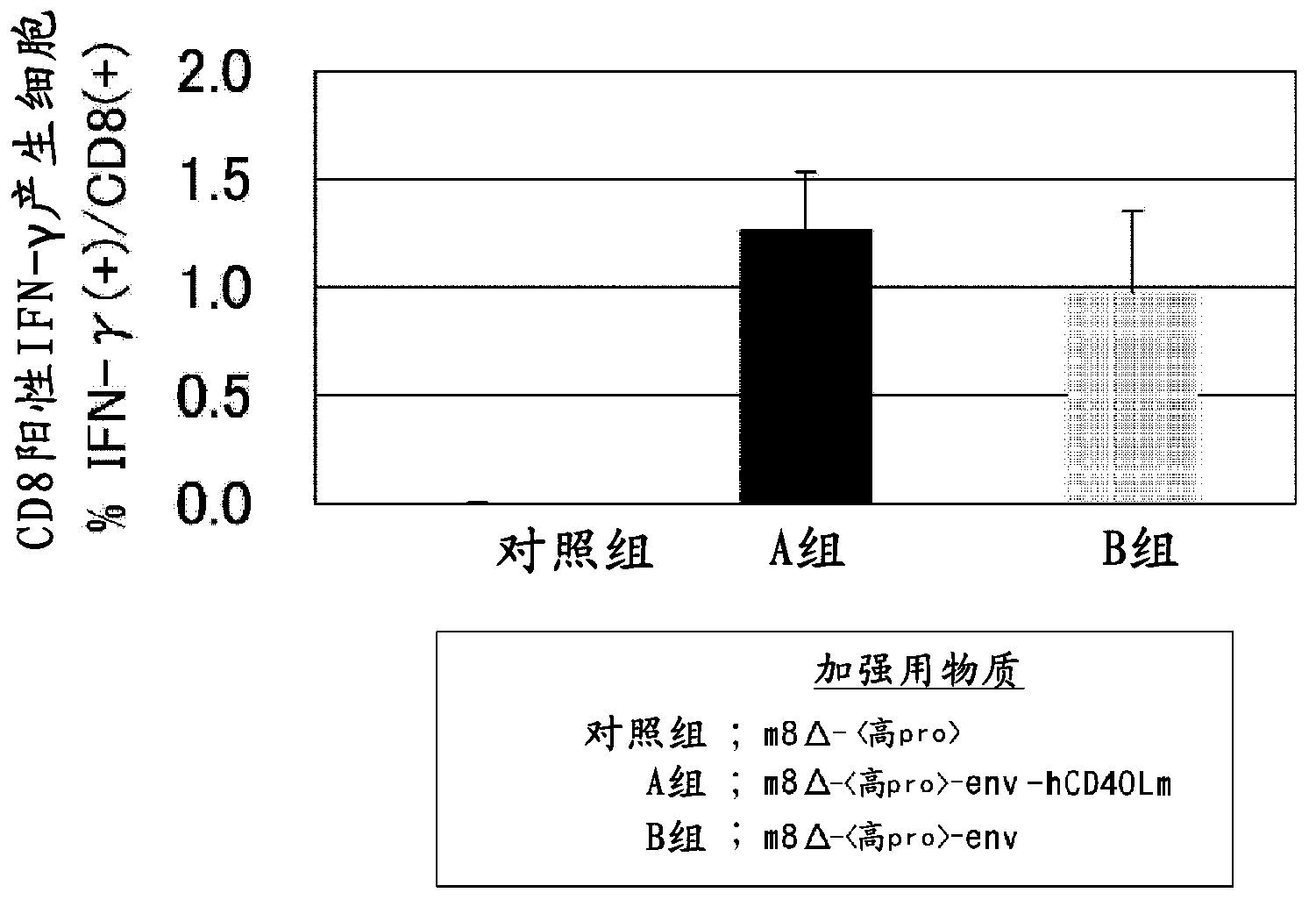
Virus vector for prime/boost vaccines, which comprises vaccinia virus vector and sendai virus vector
A technology of vaccinia virus vector and virus vector, which is applied in the direction of virus/bacteriophage, medical preparations containing active ingredients, viruses, etc., and can solve the problem that it is difficult to completely eliminate side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] (1) Preparation of vaccinia virus vector carrying the gene encoding human immunodeficiency virus envelope protein
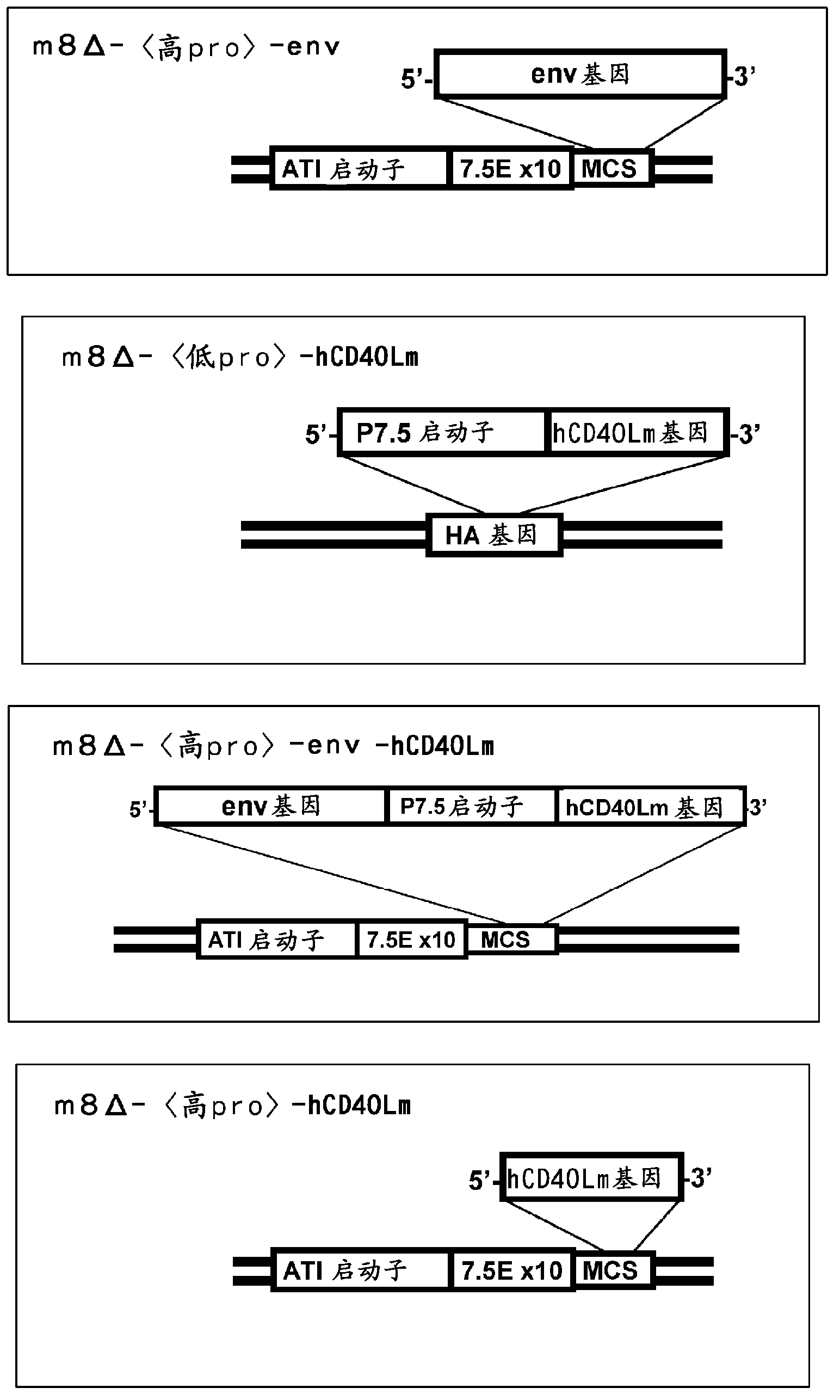
[0080] [1-1] Preparation of m8Δ--env
[0081] Preparation of env gene
[0082] pJW322-env was obtained by inserting the genes encoding the envelope proteins gp160 and gp120 (env) of the human immunodeficiency virus HIV-1 JR-CSF strain (accession number M38429) into the Avr II / Xho I site of pJW322. Then, in order to efficiently express env, the transcription termination sequences of vaccinia virus present at positions 6751 to 6757, 7367 to 7373, and 8305 to 8311 in the env gene were subjected to in vitro mutagenesis (in vitro mutagenesis) method, mutated as described below, and this was designated as pJW322-env2. Here, the amino acid sequence of env was not changed by this mutation.
[0083] 6751st to 6757th
[0084] Before mutation: TTTTTAT (sequence number 1)
[0085] After mutation: TTTCTAT (SEQ ID NO: 2)
[0086] 7367th to 7373rd
[0087] Before...
Embodiment (1
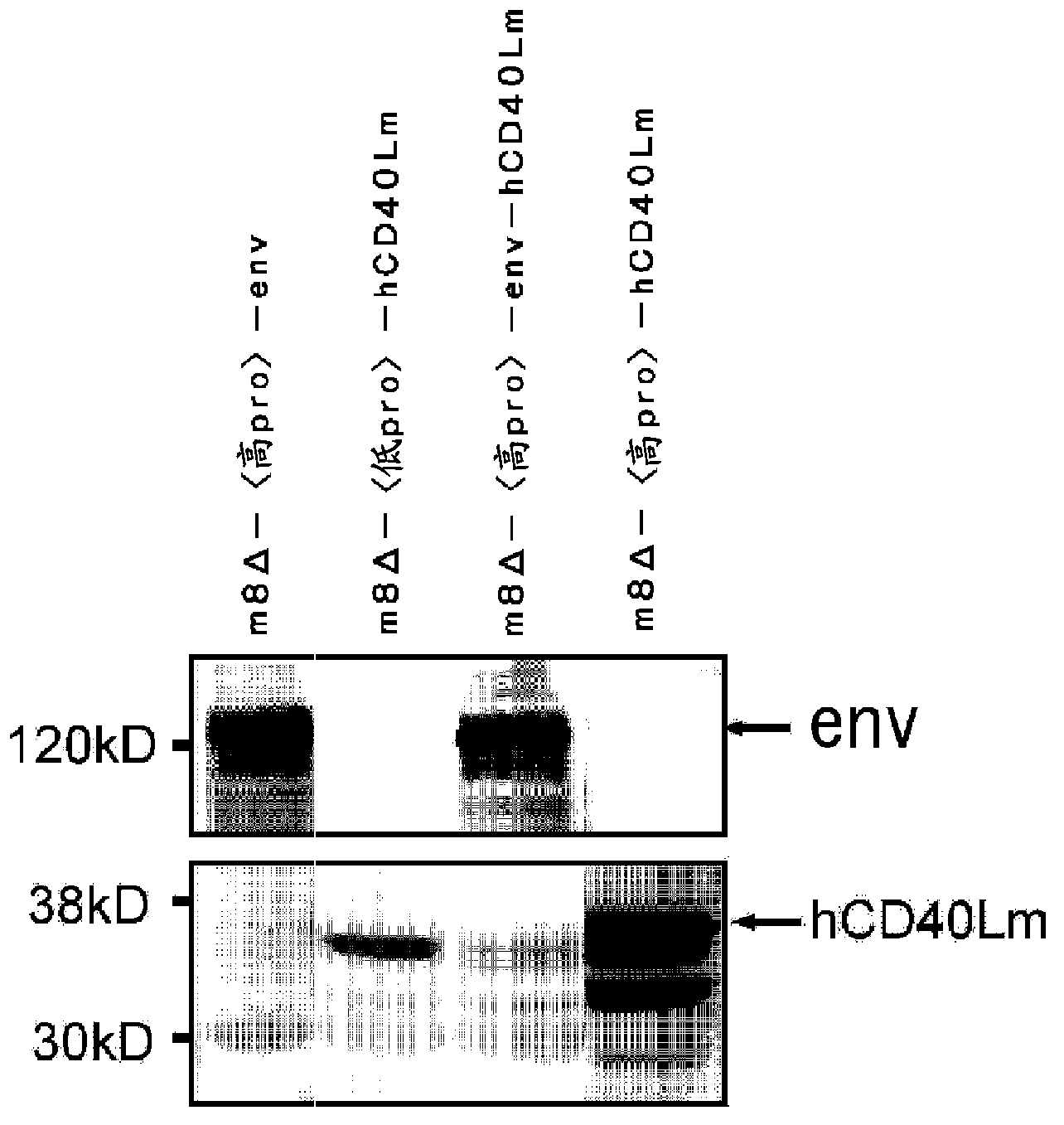
[0124] For the m8Δ--hCD40Lm of the present embodiment (1) [1-2], it is described in the present embodiment (1)[1-1] Western blotting was performed according to the method, and the expression of hCD40Lm was confirmed. Only 10 μg of cells used for electrophoresis was used instead of 1 μg. In the detection of hCD40Lm, anti-CD40L mouse monoclonal antibody was used instead of serum from HIV-1 infected patients. The results are shown in figure 2 .
[0125] Large-scale cultivation of vaccinia virus vector
[0126] By the method described in this example (1) [1-1] , the m8Δ--hCD40Lm of this example (1) was mass-cultured and purified Concentrate and determine the virus titer.
[0127] Preparation of [1-3]m8Δ--env-hCD40Lm
[0128] Preparation of plasmid
[0129] Using the pJW322-env2 of this example (1) [1-1] as a template, PCR was performed with the following primers to amplify and isolate the env gene.
[0130] Forward primer:
[0131] 5'-CTAGAATTCGCCACCATGAGAGTGAAGGGGATCAG...
Embodiment (1)
[0150] For the m8Δ--env-hCD40Lm of this embodiment (1) [1-3], through this embodiment (1)[1-1] ELISA was performed according to the method described in , and the expression of env was confirmed in the plaques. In addition, for m8Δ--env-hCD40Lm of this embodiment (1) [1-3], through this embodiment (1)[1-1] and the method described in this example (1) [1-2] performed western blotting to confirm the expression of env and hCD40Lm. The results are shown in figure 2 .
[0151] Large-scale cultivation of vaccinia virus vector
[0152] By the method described in this example (1) [1-1] , the m8Δ- >-env-hCD40Lm was purified and concentrated, and the virus titer was determined.
[0153] [1-4] Production of m8Δ--hCD40Lm
[0154] Preparation of plasmid
[0155] Using pCA-hCD40Lm3 inserted with the hCD40Lm gene as a template, PCR was performed with the following primers to amplify and isolate the hCD40Lm gene.
[0156] Forward primer: 5'-AAACCCGGGCATGATCGAAACATACAACCAAA-3' (SEQ ID...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com