Mutated amyloid protein
A technology for amyloid and protein, applied to peptide/protein components, anti-animal/human immunoglobulins, genetic material components, etc., can solve problems such as weakened side effects and undetermined improvement methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Determination of base sequence of deletion variant APP
[0107] Genomic DNA was extracted from the blood of patients with familial Alzheimer's disease, and using the obtained DNA as a template, based on the currently known disease-causing genes Presenile Gene 1 (PSEN1), Presenile Gene 2 (PSEN2) and APP, the encoding After the full base sequence of the cDNA was amplified by PCR, it was analyzed by ABI company PRISM 310 sequencer (Perkin-Elmer, CA). As a result, a mutation of one site (deletion of 3 bases gaa at positions 1852-1854 of SEQ ID NO: 1) was found in the amyloid protein coding region (SEQ ID NO: 1) of the wild-type APP gene cDNA.
Embodiment 2
[0109] Determination of the Structure of Amyloid Protein Synthesized from Deletion Mutant APP Gene cDNA
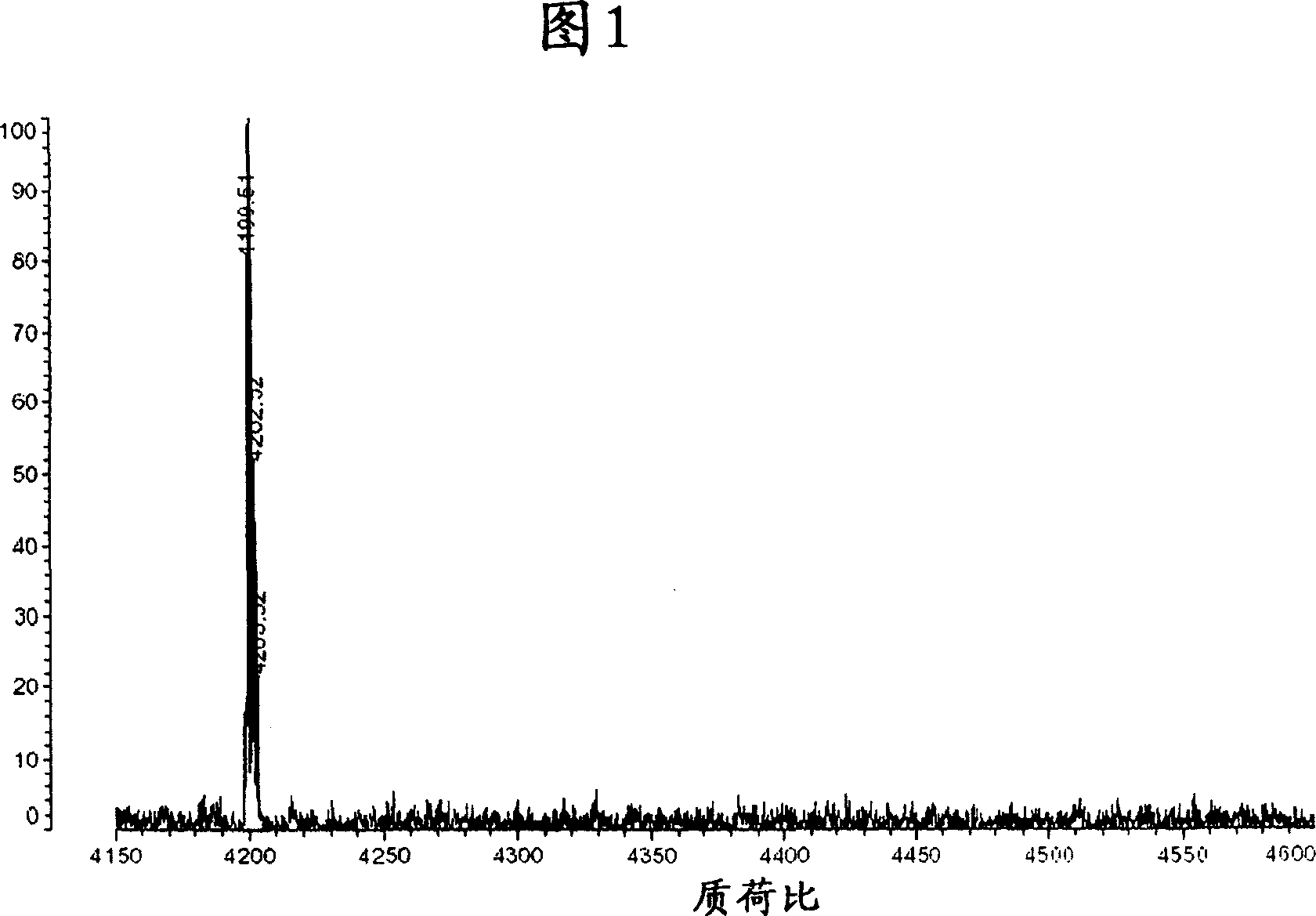
[0110] The mutated APP gene cDNA obtained in Example 1 was studied with the cleavage site of β-protein secretase or γ-protein secretase because of the variation in the amyloid protein. Then, APP is expressed, and the expression vector of mutated APP gene cDNA is introduced into human cultured HEK cells known to produce and secrete amyloid protein. After 2 days of expression, the culture supernatant was obtained, and the fraction immunoprecipitated with an anti-amyloid antibody was analyzed in Shimadzu AXIMA-CFR. The results are shown in Figure 1.
[0111] As shown in FIG. 1 , the experimentally observed value of the molecular weight of Aβ1-40 (ΔE), which is the amyloid protein secreted by the mutated APP gene cDNA expressed in human cultured HEK cells, was 4200.51. This is roughly consistent with the theoretically calculated molecular weight of 4200.69 for the Glu that l...
Embodiment 3
[0113] Quantification of amyloid protein synthesized from deletion variant APP
[0114] It was proved that the deletion mutant APP expressed in human cultured HEK cells produced and secreted two kinds of mutant amyloid proteins roughly the same as wild-type APP as shown in Table 1.
[0115] Aβ42 (pg / ml)
[0116] It should be noted that the control in Table 1 refers to the use of an empty expression vector dissolved in physiological saline. In addition, Aβ42 represents Aβ1-42 and Aβ1-43 or Aβ1-42(ΔE) and Aβ1-43(ΔE), and Aβ40 represents Aβ1-40 or Aβ1-40(ΔE).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com