Method for detecting GI type Norovirus in seafood
A virus and food technology, applied in the biological field, can solve the problems of false negatives, strong norovirus infectivity, and difficulty in detecting variants or genotypes, achieving low false positives and false negatives, wide application prospects, and sensitivity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, real-time fluorescent PCR primer design
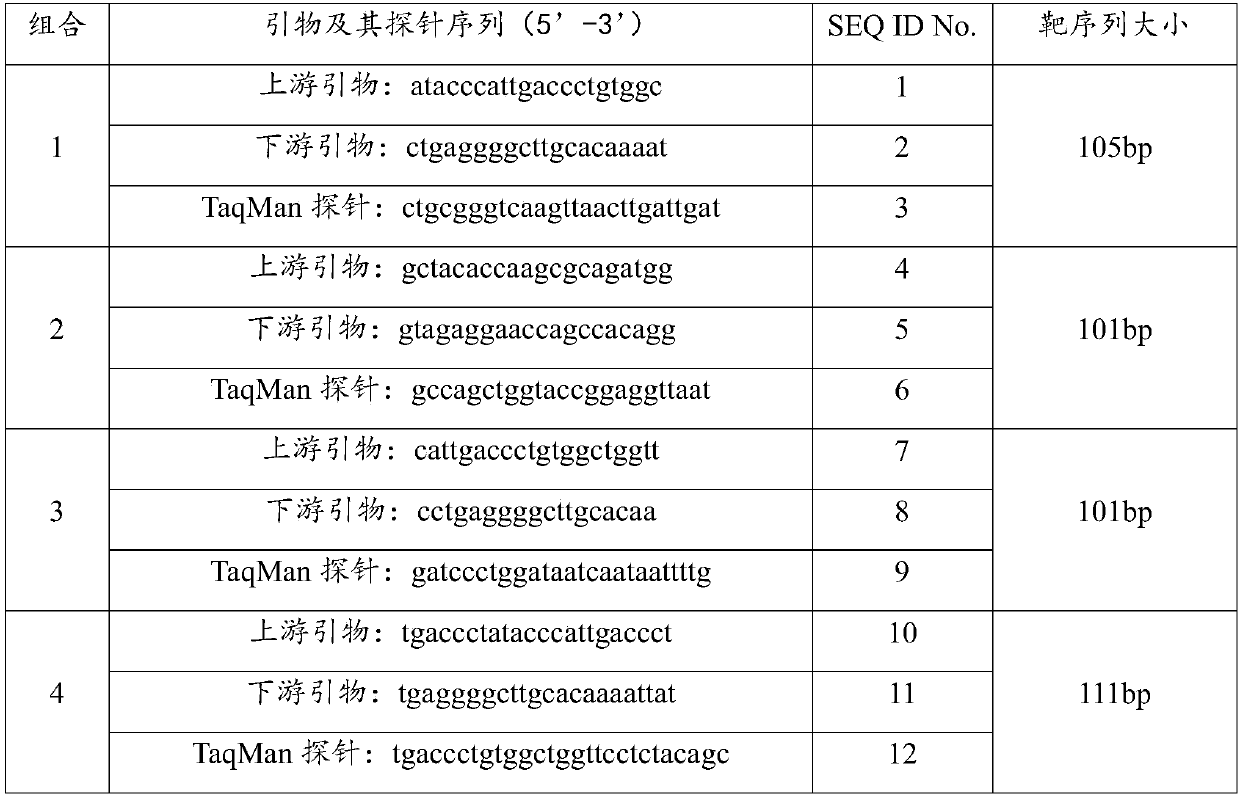
[0032] The VP1 gene sequence information of all type GI noroviruses was retrieved from NCBI. After sequence analysis and comparison, the highly conserved regions of type GI noroviruses were found, 10 pairs of primers and probes were designed, and BLAST sequence comparison was performed. , screen out 4 pairs of primers and their probe combinations with better specificity, and then carry out the biosynthesis of the sequence, in which, the 5' end of each probe is labeled with a fluorescent reporter group FAM, and the 3' end is labeled with a quencher group BHQ1, each combined sequence is shown in Table 1.
[0033] Table 1. Sequences of primers and their probe combinations
[0034]
Embodiment 2
[0035] Embodiment 2, real-time fluorescent PCR detects the specificity of GI type norovirus
[0036] Use 4 pairs of primers and probe combinations thereof shown in Table 1 in Example 1 to carry out real-time fluorescent PCR detection of oyster samples infected by the following different viruses: rotavirus, astrovirus, hepatitis A virus, different subtypes GI. GI Noroviruses from 1 to 9, GII Noroviruses of different subtypes GII.1 to 21, GIII to VII Noroviruses, each sample was repeated three times, and the results were as follows:
[0037] The detection results of blank control and negative control using different primers and their probe combinations were all negative;
[0038] The detection results of primers and probe combinations 1 and 2 in Table 1 are positive to all different subtypes of GI type norovirus strain infection samples, while other samples are negative;
[0039] The primers and their probe combinations 3 in Table 1 were positive for all different subtypes of G...
Embodiment 3
[0053] Embodiment 3, real-time fluorescence PCR detects the sensitivity of GI type norovirus
[0054] With the DNA solution of the sample identified as GI type Norovirus positive in Example 2, measure the value of OD260 / OD280, and calculate the DNA concentration, use RNase-free Water to dilute to a certain concentration, and then serially dilute to a concentration of 1 pg respectively / μL, 0.1pg / μL, 0.01pg / μL, 0.005pg / μL, 0.001pg / μL of different standard samples.
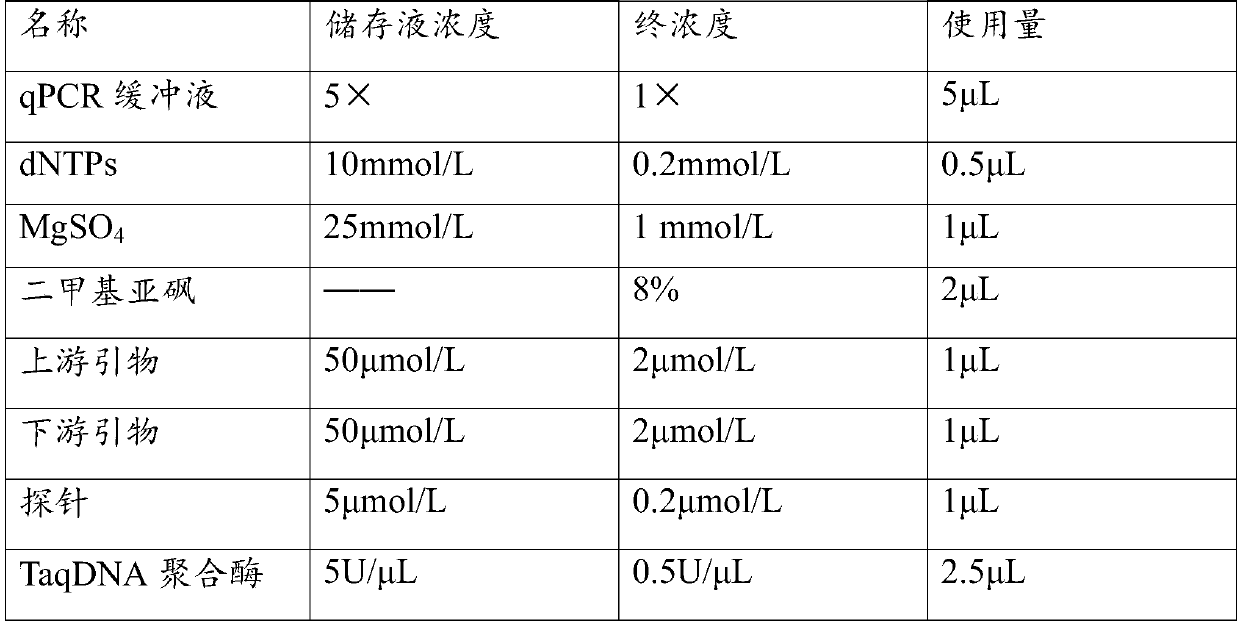
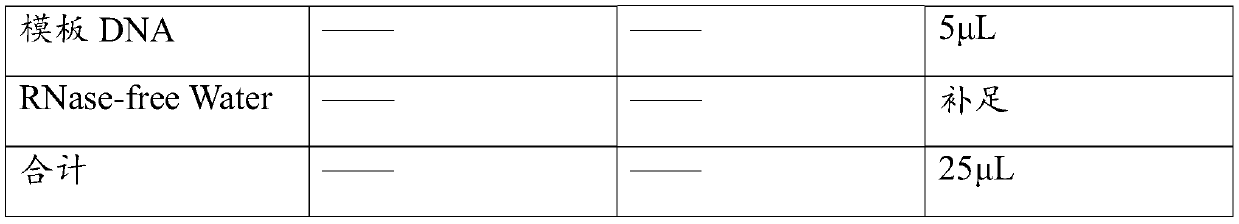
[0055] Using the above-mentioned different standard samples as templates, the primers and their probe combinations 1 and 2 in Table 1 were used respectively, and the detection was carried out according to the method of step 2 in the real-time fluorescent PCR detection described in Example 2.
[0056] Result: when the standard sample concentration of the primers and probe combination 1 in Table 1 is 0.005pg / μL and above, the detection result is positive, and when the standard plasmid concentration is 0.001pg / μL, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com