Codon-optimized EV71 VP1 gene and nucleic acid vaccine
A technology of codon optimization and nucleic acid vaccine, which is applied in the field of EV71 VP1 gene and its nucleic acid vaccine, can solve the problems of gene cloning difficult to express effectively, stimulating the host immune system, and low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
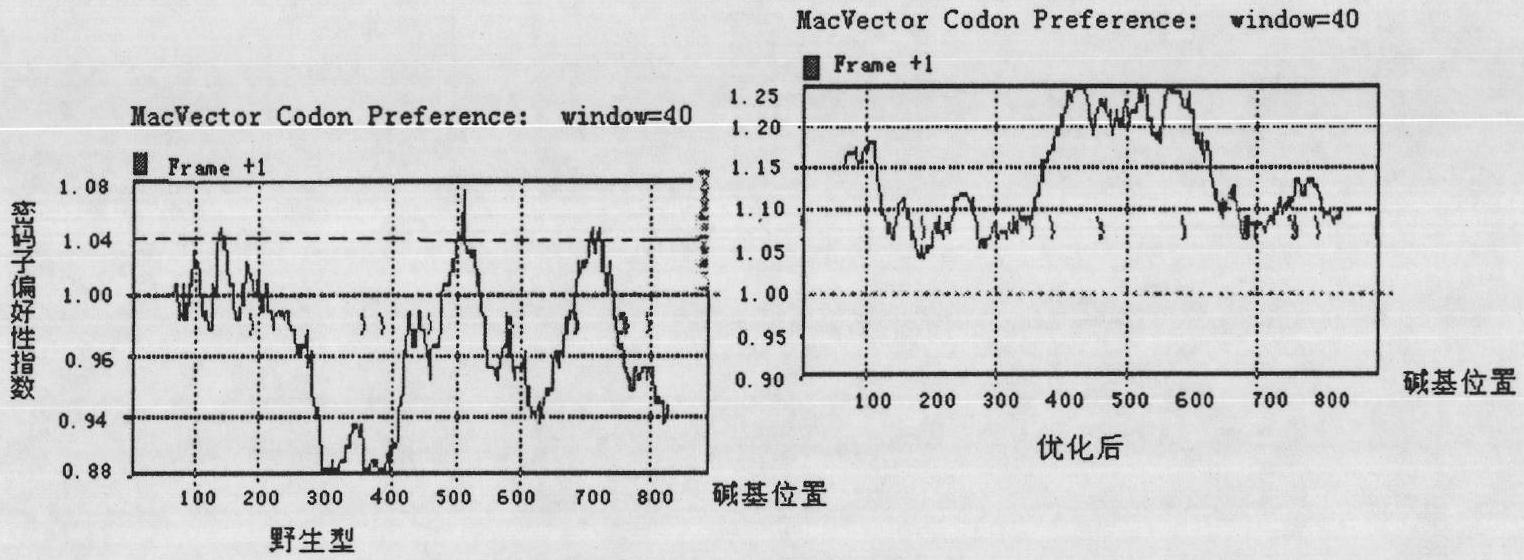
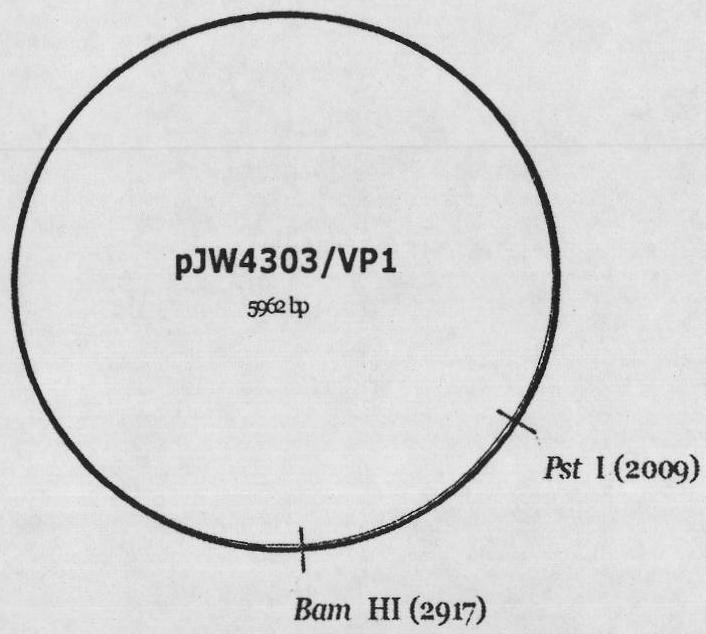
[0061] Example 1. Construction and Identification of Nucleic Acid Vaccine pJW4303 / VP1
[0062] 1.1 Preparation of Competent Escherichia coli HB101
[0063] 1.1.1 Thaw Escherichia coli HB101 stored in glycerol on ice, inoculate 5ul into 5ml LB medium, place in a constant temperature incubator at 37°C, 180rpm, and shake the bacteria overnight.
[0064] 1.1.2 Under sterile conditions, take 1ml of the overnight culture solution, inoculate it into 200ml of fresh LB medium, place it in a constant temperature incubator at 37°C, 180rpm, and shake for 2 hours.
[0065] 1.1.3 Cool the bacteria in the previous step on ice for 15 minutes.
[0066] 1.1.4 Aseptically dispense into 50ml centrifuge tubes (autoclaved), centrifuge at 2500rpm at 4°C for 15min.
[0067] 1.1.5 Add cold 0.1M Cacl 2 100ml (Cacl 2 have been autoclaved), resuspend the bacteria, and incubate on ice for 30 min.
[0068] 1.1.6 Aseptically dispense into 50ml centrifuge tubes (autoclaved), centrifuge at 2500rpm at 4°...
Embodiment 2
[0105] Example 2. Construction and Identification of Nucleic Acid Vaccine pJW4303 / tPA-VP1
[0106] 2.1 Design PCR primers, respectively: upstream primer SEQ ID NO.5, downstream primer SEQ ID NO.6; using the recombinant plasmid pGA4 / VP1 as a template, amplify the GCTAGC (NheI) and GGATCC (BamHI) VP1 gene fragment of restriction site. The reaction conditions are: 94°C for 4min, 94°C for 1min, 56°C for 1min, 72°C for 1.5min, a total of 25 cycles, and 72°C for 7min.
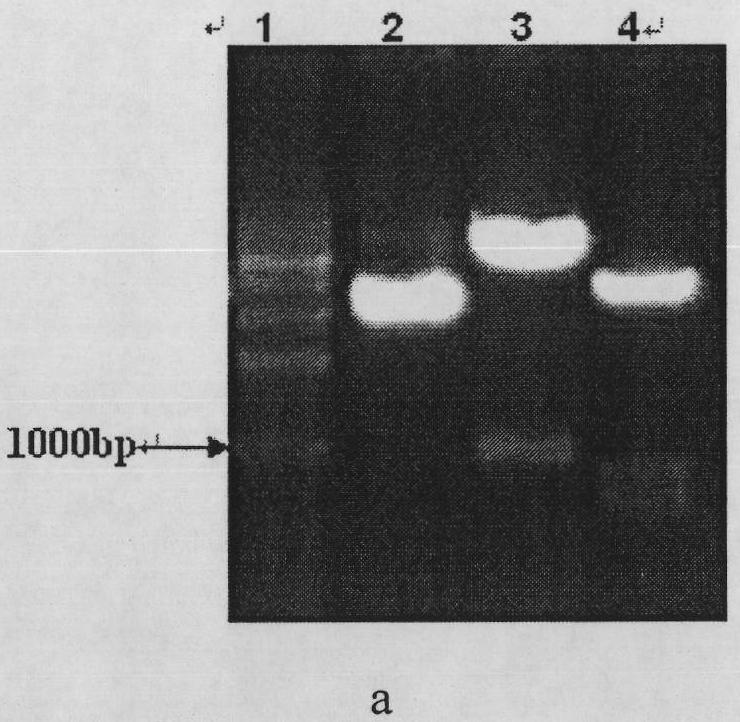
[0107] 2.2 Take 2ul of the PCR product and run 1% agarose gel electrophoresis for identification.
[0108] 2.3 Identify the correct PCR product with DNA gel recovery kit (E.Z.N.A. TM Gel Extraction Kit (50), OMEGA BIO-Tek Company) recovered and purified 914bp VP1 gene fragment. The specific steps of glue recovery are as 1.4 operation process.
[0109] 2.4 Purified PCR product and vector pJW4303 were double digested with NheI and BamHI respectively; enzyme digestion system:
[0110] 10x buffer(with BSA) 5.0ul...
Embodiment 3
[0137] Construction and Identification of Example 3.pJW4303 / tPA-VP1-tandem Nucleic Acid Vaccine
[0138] 3.1 Design two pairs of PCR primers, both of which use the recombinant plasmid pGA4 / VP1 as a template, use the upstream primer SEQ ID NO.5, and the downstream primer SEQ ID NO.7 to amplify and amplify containing GCTAGC (NheI) and GGTACC (KpnI) VP1 gene fragment 1 of restriction site; Use upstream primer SEQ ID NO.8 and downstream primer SEQ ID NO.7, amplify containing GGTACC (KpnI) and GGATCC (BamHI) VP1 gene fragment 2 of restriction site. The PCR reaction conditions are the same as those in 2.1.
[0139] 3.2 Take 2ul of the PCR product and run 1% agarose gel electrophoresis for identification.
[0140] 3.3 Identify the correct PCR product with DNA gel recovery kit (E.Z.N.A. TM Gel Extraction Kit (50), OMEGABIO-Tek Company) recovered and purified 914bp VP1 gene fragment 1 and 918bp VP1 gene fragment 2. The specific steps of glue recovery are as 1.4 operation pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com