Recombinant virus for preventing viral myocarditis as well as vaccine and applications tof recombinant virus
A technology of recombinant virus and stomatitis virus, applied in the direction of antiviral agent, virus/phage, recombinant DNA technology, etc., can solve the problem of poor effect of viral myocarditis, achieve prevention of viral myocarditis, enhance induction, improve viral Myocarditis effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Hela cell culture and CVB3 virus passage:
[0042] The human cervical cancer cell line Hela cell of the present invention is cultivated according to a conventional method, with RPMI-1640 medium containing 10% NBS, 2mM L-glutamine, 100U / ml penicillin and kanamycin sulfate at 37°C, 5% CO 2 cultured under the same conditions and passaged every other day. Infect about 5×10 6 Hela cells, after 40 hours of culture, 80% of the cells were lysed by the replicating virus, and the liquid and cell debris were centrifuged at 3000 rpm / min for 20 minutes, and the obtained supernatant was fresh CVB3 suspension.
Embodiment 2
[0043] LD of embodiment 2 CVB3 virus 50 (LD50) titration:
[0044] BALB / c suckling mice born for 48 hours were taken out and divided into 8 groups with 6 mice in each group. -1 、10 -2 、10 -3 、10 -4 、10 -5 、10 -6 、10 -7 、10 -8 , respectively take 100 μl and inject 8 groups of suckling mice through intraperitoneal cavity, observe the survival situation of suckling mice every day, and calculate the LD of this batch of CVB3 virus according to the routine method of virology 50 potency. Distance ratio = (>50% death percentage - 50) / (>50% death percentage - 50 => 50% of the logarithm of the mortality dilution (Log) + distance ratio
[0045] LD of CVB3 in the same batch in this test 50 Valence is 10 -5.5 / 100ul.
Embodiment 3
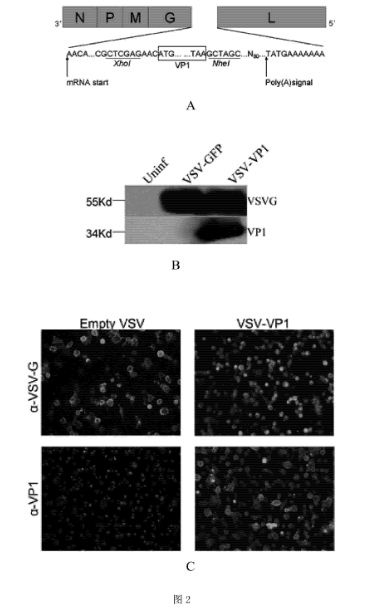
[0046] Example 3 Construction of recombinant VSV virus and identification of expression function in vitro
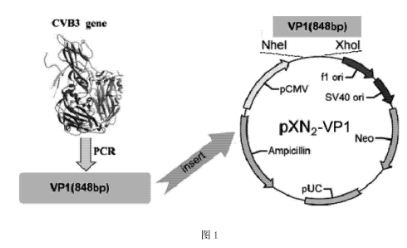
[0047] 1 Construction of the target antigen plasmid pXN2-VP1: the pXN2-VP1 plasmid with pXN2 as the carrier was constructed with the CVB3 structural protein VP1 as the target antigen. Wherein, the VP1 cDNA sequence is based on literature (Klump WM, et al. J Virol, 1990, 64(4):1573-1583), specifically shown in SEQ ID NO:1.
[0048] 1) The CVB3VP1 gene was obtained from freshly cultured CVB3 (Nancy strain) by RT-PCR.
[0049] Design the upstream and downstream primers of VP1: KV1: 5′-CCCAAGCTTGCCACCATGGGCCCAGTG GAAGACGCG-3′; KV2: 5′-CGGGATCCTTACTAAAATGCGCCCGTA TTTGT C-3′. Viral RNA was extracted according to the RNAex Reagent&System Kit of Shanghai Huashun Biological Company, and the total RNA of CVB3 was mixed with 20 μl DEPC H 2 dissolved in O and stored at -80°C.
[0050] The reverse transcription of cDNA was carried out according to the 2-N First Strand cDNA Synthes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com