Primers, probes, kit and method for synchronous amplification detection of HCMV, HSV1, HSV2 and B19
A kit and probe technology, which can be used in microorganism-based methods, biochemical equipment and methods, introduction of foreign genetic material using vectors, etc., and can solve problems such as unreported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
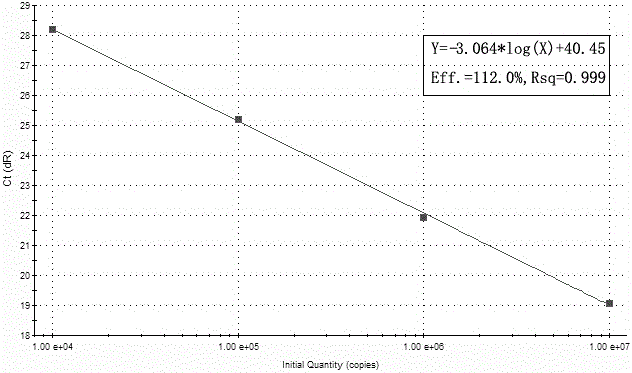
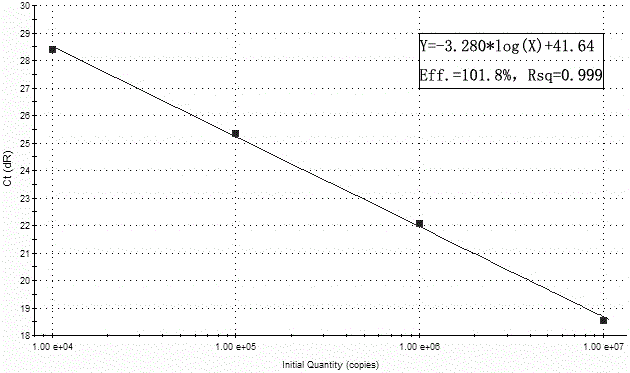
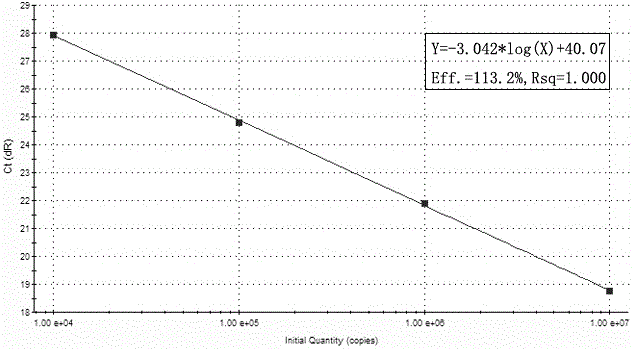
[0084] Example 1: Preparation of standard curves for cytomegalovirus, herpes simplex virus types 1 and 2 and parvovirus B19 using kits.
[0085]Establishment of standard curve for multiple detection kits (take Agilent's mx3000p as an example)
[0086] Material:
[0087] a. 4 μL of 10×PCR buffer, 5U / μL Taq DNA polymerase 0.5 μL, 25mM MgCl 2 3.0 μL, 10 mM dNTPs 1.0 μL, 10 μmol / L HCMV, HSV1, HSV2 and B19 upstream and downstream primers 0.8 μL each, 10 μmol / L HCMV, HSV1, HSV2 and B19 probes 1.6 μL each, template DNA 2 μL, no Bacterial double distilled water 16.7μL, the total reaction solution is 40μL;
[0088] b. Standard positive template stock solution: the concentration is 10 7 Copy / μL standard positive template, and then perform 10-fold serial dilution;
[0089] c. Negative quality control standard: sterile double distilled water.
[0090] method:
[0091] A. Serially dilute the positive standard template to 10 7 copies / μL, 10 6 copies / μL, 10 5 copies / μL, 10 4 copie...
Embodiment 2
[0094] Example 2: An example of detection of clinical samples by a multiplex detection kit (taking Agilent's mx3000p as an example)
[0095] Take 38 μL of the fluorescent quantitative PCR reaction solution, 2 μL of the positive standard template, and set a negative control, respectively add to different PCR reaction tubes, and perform PCR detection in parallel on the fluorescent quantitative PCR instrument. The cycle conditions are: 50°C for 2min, 94°C for 30s, and the cycle process uses a two-step method of 94°C for 5s, 60°C for 1min, 40 cycles. Simultaneous detection of FAM, HEX, ROX and CY5. After the cycle is over, use the software that comes with the instrument to read the results. See Figure 6 , the amplification curves of the positive standard and clinical samples present a smooth S-shape, indicating that the amplification efficiency is good. Virus quantification results can be calculated according to the standard curve in Implementation 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com