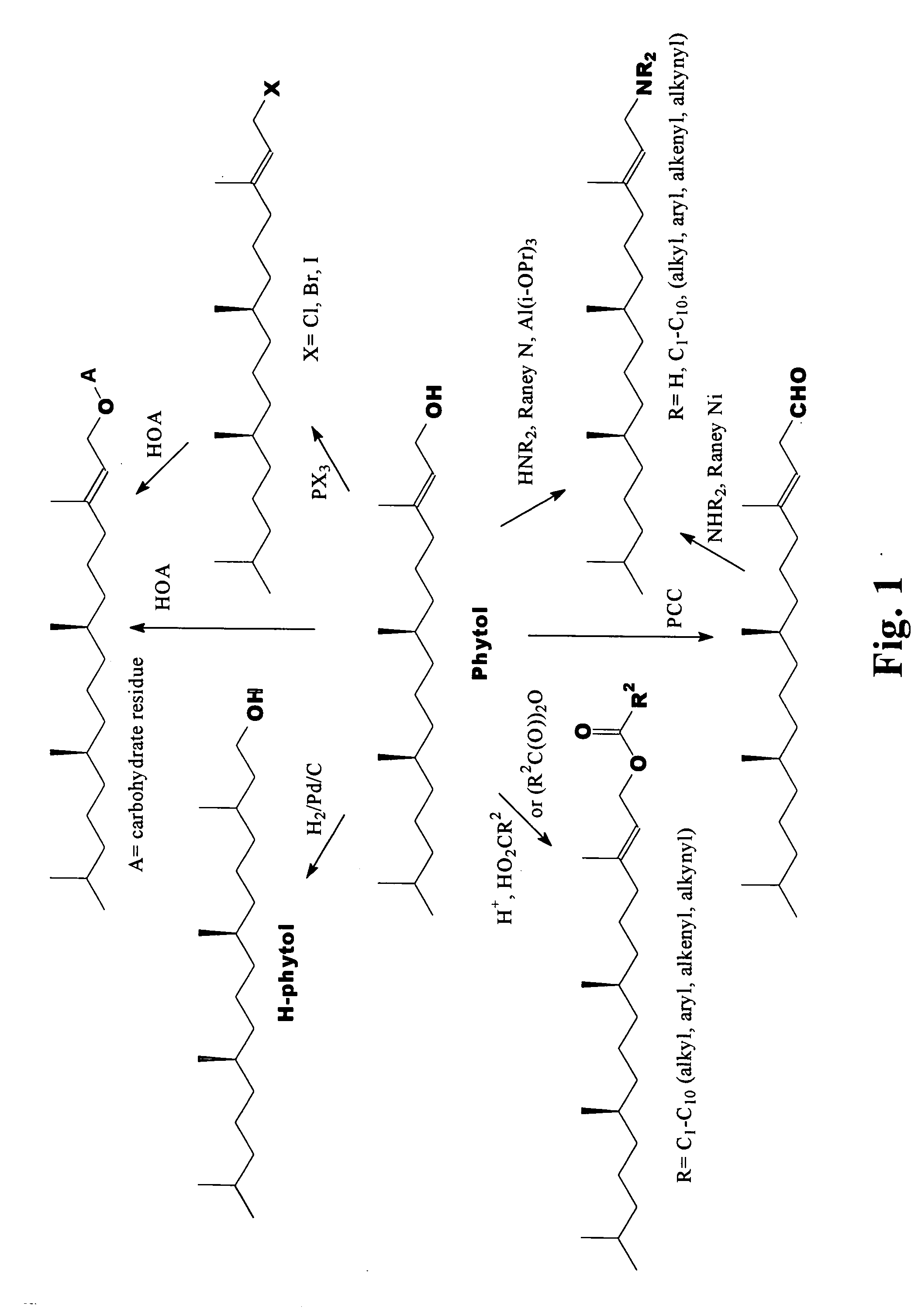
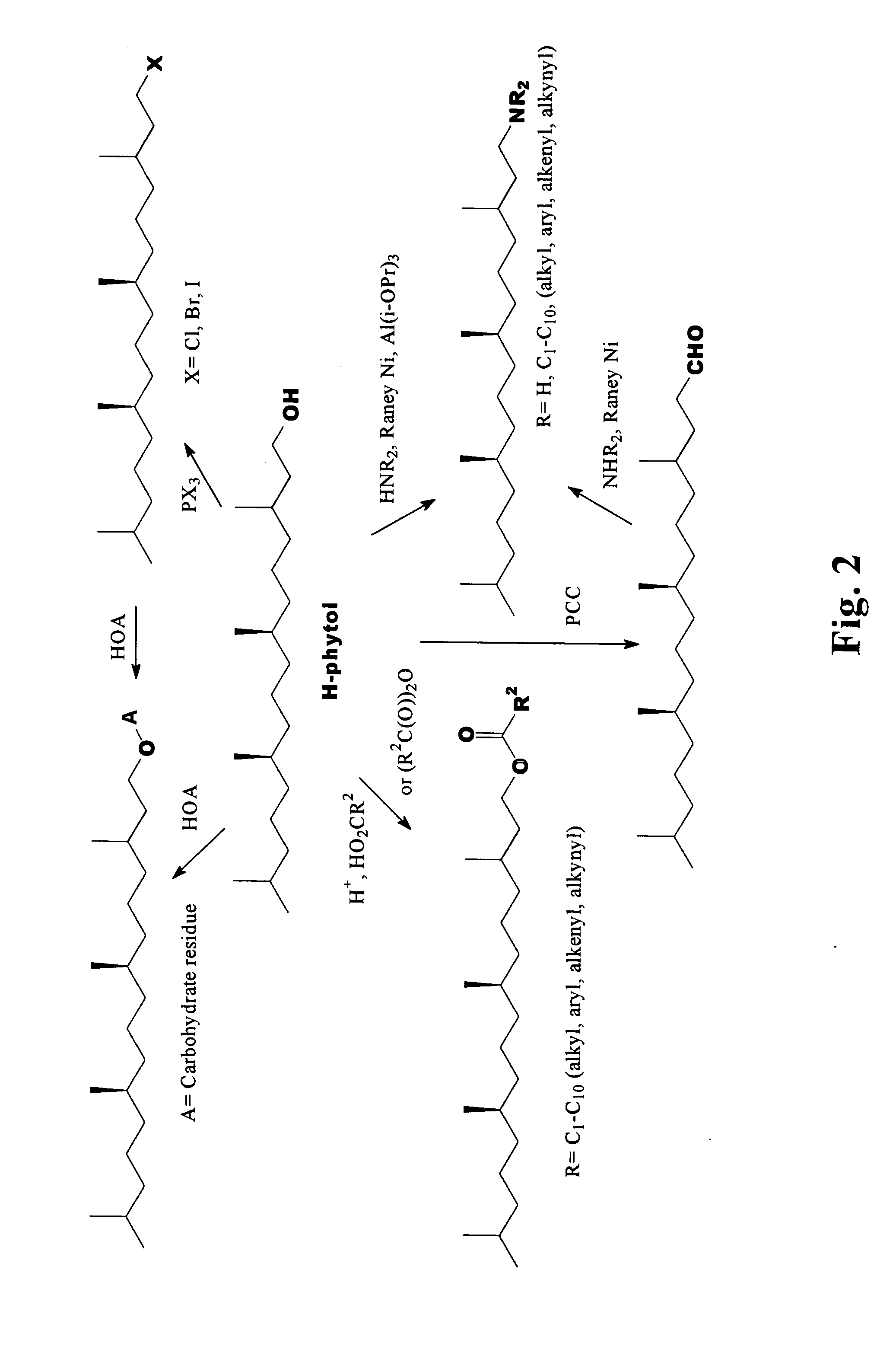
Novel phytol derived immunoadjuvants and their use in vaccine formulations
a vaccine formulation and immunoadjuvant technology, applied in the field of immunoadjuvants, can solve the problems of inducing severe side reactions, severe adverse inflammatory reactions, and toxic adjuvants, and achieve the effect of enhancing the immunogenicity of a vaccine composition and inducing the immunogenic respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 2,3-Dihydrophytol (phytanol or H-phytol)
[0043]
[0044] To a solution of 1.00 g phytol in dry tetrahydrofuran was added 0.10 g 5% palladium on carbon. The mixture was allowed to stir 24 h under hydrogen at 1 atm. The catalyst was removed by centrifugation and the solvent was then removed by rotary evaporation. The resulting oil was purified by molecular distillation at about 100° C. and 0.1 mm Hg to give a colorless oil: 1H NMRδ (ppm) 0.90 (d, ˜15H), 1-1.8 (m, ˜25H), 3.7 (t, 2H).
[0045] Hydrogenation of phytol can also be accomplished using Raney nickel and hydrogen to yield phytanol as referenced in Bendavid, A., Burns, C. J., Leslie, D. F., Hashimoto, K., Ridley, D. D., Sandanayake, S., and Wieczorek L., J. Org. Chem. (2001), 66, 3709-3716; Kim, T., Chan, K. C, and Crooks, R. M., J. Am. Chem. Soc. (1997) 119, 189; and Jellum, E., Eldjarn, L., and Try, K., Acta Chem. Scand. (1966), 20, 2335.
example 2
Preparation of Halogenated Phytol / Phytanol
[0046] Hologenated phytol can be prepared according the procedure outlined below. The procedure shown below is a general one and can be found in the above references, and in particular in Bendavid, A., Burns, C. J., Leslie, D. F., Hashimoto, K., Ridley, D. D., Sandanayake, S., and Wieczorek, L., J. Org. Chem. (2001), 66, 3709-3716.
example 3
Preparation of Phytanyl Glucose / Phytanyl Mannose
[0047] Phytanyl glucose has been previously reported (1, 2). The procedure used by Hato and coworkers (2) to make this compound in moderate yield is the same procedure that they used to make some similar acetals several years earlier (3). However, recent reports (4, 5, 6) of the preparation of some similar lipidated carbohydrate acetals appear to offer even better ways of making phytanyl glucose. Hinguchi and coworkers (4) converted glucose acetate to a bromide by treating with HBr and then converted this to an acetal by treating it with a long-chain alcohol, AgClO4 and AgCO3. It is envisioned that a procedure, as shown below, can be used with phytanol.
[0048] Alternatively, a phytanyl glucose conjugate can be prepared by adapting a procedure reported by Clausen and coworkers (glucose acetate, ROH, BF3—OEt2, CH2Cl2) (5) or by the procedure disclosed by Konstantinovic and coworkers (glucose acetate, ROH, SnCl4) (6). A similar scheme c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| compositions | aaaaa | aaaaa |
| weight ratio | aaaaa | aaaaa |
| hydrophilic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com